Synthesis of cyclic 1,3-diols as scaffolds for spatially directed libraries†
Abstract
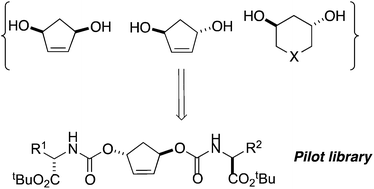
A useful design element in small molecule libraries is spatial diversity, in which binding moieties are systematically directed toward different regions of three-dimensional space. One way of achieving this is through the use of conformationally diverse scaffolds onto which various binding moieties can be placed. Such scaffolds can represent synthetic challenges of their own. In this paper, we describe a new route to chiral, cyclic 1,3-diol building blocks that features silylated dithianes as relay linchpins. These diols are subsequently used for the construction of a 24-compound pilot library bearing two amino acid residues.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery
 Please wait while we load your content...
Please wait while we load your content...