Themed collection CO2 capture and conversion

Introduction to CO2 capture and conversion
Elena Shevchenko, Ah-Hyung Alissa Park, Shouheng Sun and Tierui Zhang introduce the Nanoscale themed collection on CO2 capture and conversion.

Nanoscale, 2023,15, 855-858
https://doi.org/10.1039/D2NR90219B
A review on ZnS-based photocatalysts for CO2 reduction in all-inorganic aqueous medium
This article reviews the strategies of maximizing CO2 photoreduction rates in distinguished ZnS-based photocatalytic systems by continuously optimizing the reaction medium and photocatalysts.
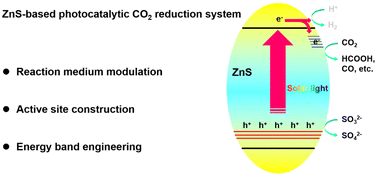
Nanoscale, 2022,14, 14455-14465
https://doi.org/10.1039/D2NR03703C
Recent progress of Bi-based electrocatalysts for electrocatalytic CO2 reduction
Both unary Bi and binary BiSn catalysts exhibit high faradaic efficiencies toward the production of formic acid via electrocatalytic CO2 reduction approach. The electrocatalytic abilities are directly related to the structures and morphologies of the metal-based catalysts.
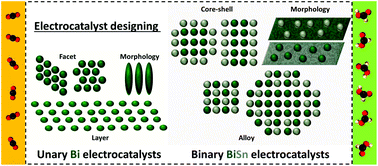
Nanoscale, 2022,14, 7957-7973
https://doi.org/10.1039/D2NR01900K
Alloying strategies for tuning product selectivity during electrochemical CO2 reduction over Cu
Alloying is efficient for tuning product selectivity of copper in electrochemical reduction of CO2. Different alloying strategies and their impacts on product formation paths, the key challenges and future directions of the field have been reviewed.
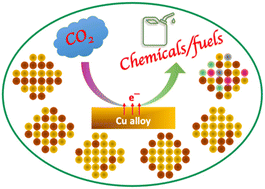
Nanoscale, 2022,14, 15560-15585
https://doi.org/10.1039/D2NR03539A
Advancing integrated CO2 electrochemical conversion with amine-based CO2 capture: a review
This review paper provides an overview of the fundamental and applied aspects of advancing carbon dioxide electrolysis for the integrated amine-based CO2 capture and conversion.
Nanoscale, 2022,14, 11892-11908
https://doi.org/10.1039/D2NR03310K
Silver based photocatalysts in emerging applications
To evaluate the role of Ag in Ag-based photocatalysts, heterojunction and localized surface plasmon resonance effect are reviewed along with emerging applications - CO2 reduction, water splitting, antibacterial application and pollutant removal, etc.

Nanoscale, 2022,14, 11909-11922
https://doi.org/10.1039/D2NR02665A
Recent advances in CO2 capture and reduction
The ever-increasing energy demand leads to fast depletion of fossil fuels and excessive CO2 emission into the atmosphere, and requires efficient capture and conversion of CO2 to achieve negative carbon emission and energy sustainability.
Nanoscale, 2022,14, 11869-11891
https://doi.org/10.1039/D2NR02894H
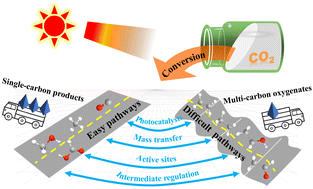
Photocatalytic CO2 conversion: from C1 products to multi-carbon oxygenates
This review focuses on the recent research progress in photocatalytic CO2 conversion systems from C1 products to multi-carbon oxygenates.
Nanoscale, 2022,14, 10268-10285
https://doi.org/10.1039/D2NR02588D
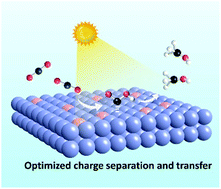
Ultrathin Ti-doped WO3 nanosheets realizing selective photoreduction of CO2 to CH3OH
Doping Ti atoms into ultrathin WO3 nanosheets can enhance the separation of photogenerated carriers and facilitate charge transfer, which is beneficial for CO2 activation and COOH* formation, thus promoting the formation of CH3OH.
Nanoscale, 2022,14, 14023-14028
https://doi.org/10.1039/D2NR02364D
Cupric porphyrin frameworks on multi-junction silicon photocathodes to expedite the kinetics of CO2 turnover
The 3D porous Cu-TCPP(Cu) MOF was adopted as the catalytic scaffold on exquisite multi-junction Si-based photocathodes for boosting both the kinetics and selectivity of photoelectrochemical CO2 reduction reactions.

Nanoscale, 2022,14, 8906-8913
https://doi.org/10.1039/D2NR01921C
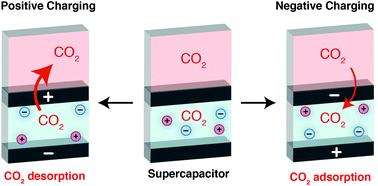
Enhancing the capacity of supercapacitive swing adsorption CO2 capture by tuning charging protocols
Electrochemical carbon dioxide capture by supercapacitors is found to depend strongly on charging protocols. Varying the charging polarity leads to increases in capture capacities and improved mechanistic understanding of the capture process.
Nanoscale, 2022,14, 7980-7984
https://doi.org/10.1039/D2NR00748G
High surface area siloxene for photothermal and electrochemical catalysis
A novel siloxene material with a high specific surface area of 217.8 m2 g−1 was prepared with a feasible room-temperature method, enabling high catalytic performances for both photothermal CO2 reduction and electrochemical hydrogen evolution.
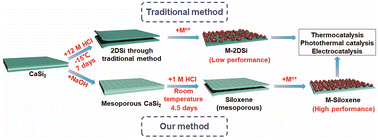
Nanoscale, 2023,15, 154-161
https://doi.org/10.1039/D2NR05140K
Ru-promoted perovskites as effective redox catalysts for CO2 splitting and methane partial oxidation in a cyclic redox scheme
The current study reports AxA′1−xByB′1−yO3−δ perovskite redox catalysts (RCs) for CO2-splitting and methane partial oxidation (POx) in a cyclic redox scheme.
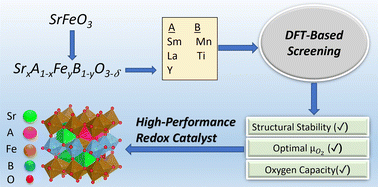
Nanoscale, 2022,14, 18094-18105
https://doi.org/10.1039/D2NR04437D
Yolk–shell-type CaO-based sorbents for CO2 capture: assessing the role of nanostructuring for the stabilization of the cyclic CO2 uptake
Yolk(CaO)–shell(ZrO2)-structured sorbents yield superior materials for high-temperature CO2 capture by mitigating deactivation via sintering and mixed phase (CaZrO3) formation.
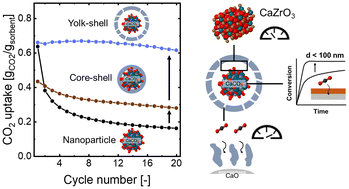
Nanoscale, 2022,14, 16816-16828
https://doi.org/10.1039/D2NR04492G
Establishing tungsten carbides as active catalysts for CO2 hydrogenation
Tungsten nanoparticles are encapsulated in silica, preserving particle size during carburization, leading to active and selective catalysts for CO2 hydrogenation.

Nanoscale, 2022,14, 16458-16466
https://doi.org/10.1039/D2NR03281C
Designing optimal core–shell MOFs for direct air capture
Metal–organic frameworks (MOFs) can selectively adsorb CO2, but are often ineffective in the presence of H2O, which binds more strongly. By selecting MOF ‘shells’ to keep water out of MOF ‘cores’ this limitation may be overcome.
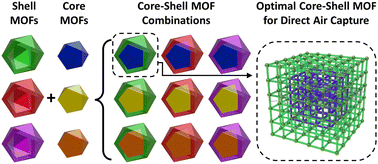
Nanoscale, 2022,14, 16085-16096
https://doi.org/10.1039/D2NR03177A
Bimetallic RuNi-decorated Mg-CUK-1 for oxygen-tolerant carbon dioxide capture and conversion to methane
A metal–organic framework, known as Mg-CUK-1, is loaded with Ru and Ni nanoparticles and evaluated as a hybrid sorbent/catalyst for the integrated capture and conversion of carbon dioxide to methane under temperature-swing operating conditions.
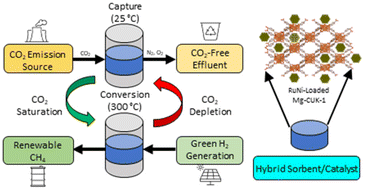
Nanoscale, 2022,14, 15669-15678
https://doi.org/10.1039/D2NR03338K
Wet flue gas CO2 capture and utilization using one-dimensional metal–organic chains
Herein we describe the use of an ultramicroporous metal–organic framework for the selective capture of carbon dioxide from wet flue gas followed by its conversion to value-added products.
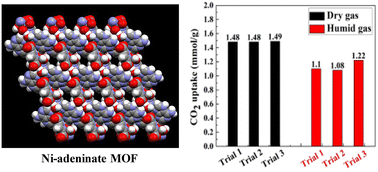
Nanoscale, 2022,14, 14962-14969
https://doi.org/10.1039/D2NR04156A
Investigating the role of potassium cations during electrochemical CO2 reduction
The specific identity of electrolyte cations has many implications in various electrochemical reactions. However, the exact mechanism by which cations affect electrochemical reactions is unknown.

Nanoscale, 2022,14, 14185-14190
https://doi.org/10.1039/D2NR03438G
Enhanced precipitation of magnesium carbonates using carbonic anhydrase
Bio-enhanced carbonate precipitation for CO2 mineralization.
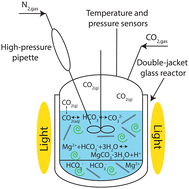
Nanoscale, 2022,14, 13570-13579
https://doi.org/10.1039/D2NR03199J
Competition between reverse water gas shift reaction and methanol synthesis from CO2: influence of copper particle size
The hydrogenation of CO2 is a structure sensitive reaction over copper nanoparticles. The particle size effect has been related to the differences in reaction intermediate coverage for different Cu facets whose abundancy vary with the particle size.
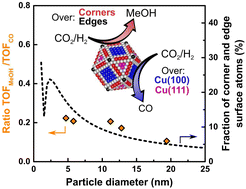
Nanoscale, 2022,14, 13551-13560
https://doi.org/10.1039/D2NR02612K
Grazing incidence X-Ray diffraction: identifying the dominant facet in copper foams that electrocatalyze the reduction of carbon dioxide to formate
GIXRD is used to determine the relative ratio of facets in porous electrocatalysts, thus providing a general technique for evaluating how the surface faceting affects product selectivity for CO2 conversion: (left) Bragg–Brentano vs. (right) GIXRD
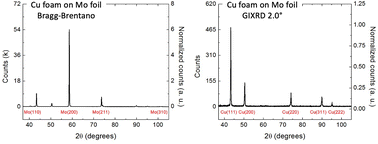
Nanoscale, 2022,14, 13132-13140
https://doi.org/10.1039/D2NR03212K
Capture and electrochemical conversion of CO2 in molten alkali metal borate–carbonate blends
CO2 captured from high temperature effluent gases by molten borate salts are reduced electrochemically to form carbon nanotubes.
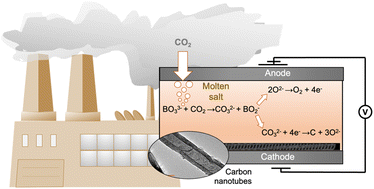
Nanoscale, 2022,14, 13141-13154
https://doi.org/10.1039/D2NR03355K
Facilitated transport membrane with functionalized ionic liquid carriers for CO2/N2, CO2/O2, and CO2/air separations
CO2 separations from cabin air and the atmospheric air are achieved by ionic liquid containing facilitated transport membrane.
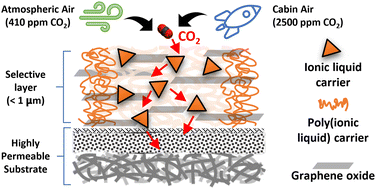
Nanoscale, 2022,14, 12638-12650
https://doi.org/10.1039/D2NR03214G
Feasibility of switchable dual function materials as a flexible technology for CO2 capture and utilisation and evidence of passive direct air capture
In this work we show that it is possible to design “switchable” dual function materials that can directly convert carbon dioxide into useful products using hydrogen or methane. These DFMs offer a means to respond to changes in the energy sector.
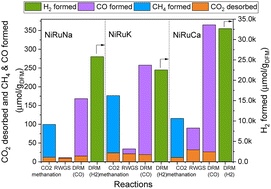
Nanoscale, 2022,14, 12620-12637
https://doi.org/10.1039/D2NR02688K
Alkyl group-decorated g-C3N4 for enhanced gas-phase CO2 photoreduction
Alkyl groups are grafted onto the surface of g-C3N4 for optimizing the adsorption of reactants and improving the photoelectronic properties, and thus greatly enhance the photocatalytic CO2 reduction activity.
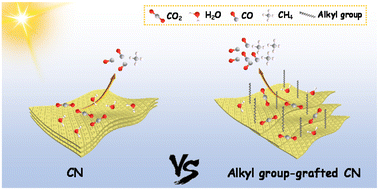
Nanoscale, 2022,14, 11972-11978
https://doi.org/10.1039/D2NR02551E
Synergistic effect of Cu and Fe small nanoparticles supported on porous N-doped graphitic framework for selective electrochemical CO2 reduction at low overpotential
Electrochemical CO2 reduction is an appealing approach to diminish CO2 emissions, while obtaining valuable chemicals and fuels from renewable electricity.
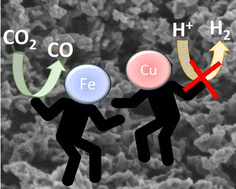
Nanoscale, 2022,14, 11583-11589
https://doi.org/10.1039/D2NR02523J
Rationally designed nanoarray catalysts for boosted photothermal CO2 hydrogenation
A combined structural engineering strategy and thinning strategy were used to optimize nanoarray-based photothermal catalysts, showing a high CO2 conversion rate of 1780 mmol gCo-1 h-1.

Nanoscale, 2022,14, 11568-11574
https://doi.org/10.1039/D2NR02680E
All-carbon microporous graphitic photocatalyst-promoted reduction of CO2 to CO in the absence of metals or dopant elements
Microporous graphitic carbon derived from cyclodextrins exhibited photocatalytic activity in CO2 reduction upon irradiation in the presence of triethanolamine, forming H2 (19 μmol h−1), CO (23 μmol h−1) and CH4 (4 μmol h−1).
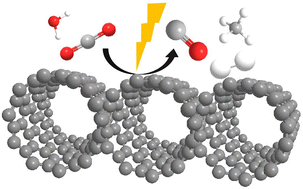
Nanoscale, 2022,14, 11575-11582
https://doi.org/10.1039/D2NR02655D
Ag@Pd bimetallic structures for enhanced electrocatalytic CO2 conversion to CO: an interplay between the strain effect and ligand effect
Pd overlayer content in Ag@Pd bimetallic nanoparticles determines the strain profile and CO2 conversion performance.
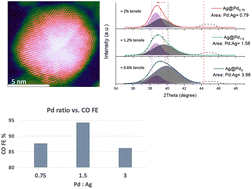
Nanoscale, 2022,14, 11187-11196
https://doi.org/10.1039/D2NR03079A
Confinement induces stable calcium carbonate formation in silica nanopores
Confinement mediates the formation of calcite preferentially over metastable carbonate phases due to the presence of fewer water molecules in the first hydration shell of calcium ions in confined fluids compared to in bulk fluids.

Nanoscale, 2022,14, 10349-10359
https://doi.org/10.1039/D2NR01834A
Structural modification of salt-promoted MgO sorbents for intermediate temperature CO2 capture
A MgO sorbent synthesized on a CNT template achieved stable multicycle CO2 capture performance by preventing agglomeration and preserving the material's structure.

Nanoscale Adv., 2022,4, 3083-3090
https://doi.org/10.1039/D2NA00213B
Electrodeposited Sn–Cu@Sn dendrites for selective electrochemical CO2 reduction to formic acid
Electrodeposited Sn–Cu@Sn dendrites have enhanced selectivity for CO2 reduction to formic acid on a gas diffusion electrode.
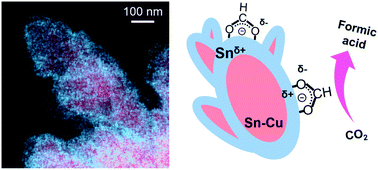
Nanoscale, 2022,14, 9297-9303
https://doi.org/10.1039/D2NR01563C
In situ probing the dynamic reconstruction of copper–zinc electrocatalysts for CO2 reduction
The result of probing the dynamic structure of co-sputtered CuO and ZnO electrocatalyst suggests the reversing Ostwald ripening of Cu structure is triggered during CO2RR and has a key impact on its methane selectivity.
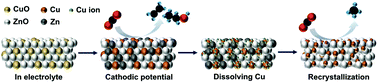
Nanoscale, 2022,14, 8944-8950
https://doi.org/10.1039/D2NR01478E
Temperature-dependent CO2 sorption and thermal-reduction without reactant gases on BaTiO3 nanocatalysts at low temperatures in the range of 300–1000 K
Low temperature CO2 reduction and mechanism on BaTiO3 nanocatalysts from 500 K, CO2 physical adsorption at 300–500 K, CO2 chemisorption above 450 K, CO2 reduction at 500–850 K, and CO2 and CO release above 800 K.
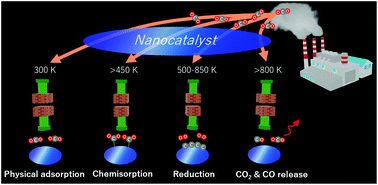
Nanoscale, 2022,14, 8318-8325
https://doi.org/10.1039/D2NR00883A
TiO2-Modulated tetra(4-carboxyphenyl)porphyrin/perylene diimide organic Z-scheme nano-heterojunctions for efficient visible-light catalytic CO2 reduction
A cascade Z-scheme nano-heterojunction of TiO2 incorporated TCPP/PDI with full spectrum response is constructed for efficient CO2 reduction, in which the energy platform TiO2 directs charge transfer by inhibiting the pathway as that in a type II one.

Nanoscale, 2022,14, 8041-8049
https://doi.org/10.1039/D2NR01753A
Boosting photocatalytic CO2 reduction via Schottky junction with ZnCr layered double hydroxide nanoflakes aggregated on 2D Ti3C2Tx cocatalyst
The ZnCr-LDH/Ti3C2Tx hybrid obtained using an in situ coprecipitation method was forming a Schottky junction to promote the yield of photoreduction CO2 to CO and CH4 gas.
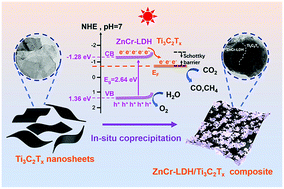
Nanoscale, 2022,14, 7538-7546
https://doi.org/10.1039/D2NR01448C
Laser-ablation assisted strain engineering of gold nanoparticles for selective electrochemical CO2 reduction
This article reports a facile laser ablation in liquid (LAL) strategy for synthesizing gold nanoparticles (Au NPs) whose rich compressive strain can greatly promote the electrochemical CO2 reduction performance of Au.
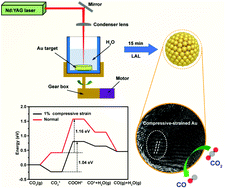
Nanoscale, 2022,14, 7702-7710
https://doi.org/10.1039/D2NR01400A
Robocasting of 3D printed and sintered ceria scaffold structures with hierarchical porosity for solar thermochemical fuel production from the splitting of CO2
We report the first ever robocast (additive manufacturing/3D printing) sintered ceria scaffolds, and explore their use for the production of renewable fuels via solar thermochemical fuel production using concentrated solar energy.
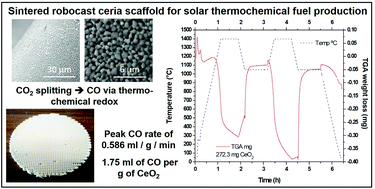
Nanoscale, 2022,14, 4994-5001
https://doi.org/10.1039/D2NR00393G
About this collection
Guest Edited by Professor A.-H. Alissa Park (Columbia University, USA), Dr Elena Shevchenko (Argonne National Laboratory, USA), Professor Shouheng Sun (Brown University, USA) and Professor Tierui Zhang (Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, China).
Understanding CO2 capture and conversion has been essential in our efforts to build a carbon neutral/negative society and to achieve energy sustainability. Recent studies have shown that CO2 can be captured from industry waste in more energy efficient manners and be converted more selectively via various catalytic processes to reusable chemicals and fuels. This collection focuses on theoretical and experimental CO2 capture and reduction through thermochemical, electrochemical, photochemical, photo/electrocatalytic, biological and inorganic carbonate-based approaches, and aims to collect the latest state-of-the-art progress made in CO2 capture and conversion into a single online collection.