Electrodeposited Sn–Cu@Sn dendrites for selective electrochemical CO2 reduction to formic acid†
Abstract
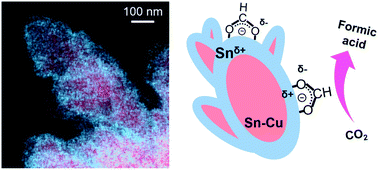
Large-scale CO2 electrolysis can be applied to store renewable energy in chemicals. Recent developments in gas diffusion electrodes now enable a commercially relevant current density. However, the low selectivity of the CO2 reduction reaction (CO2RR) still hinders practical applications. The selectivity of the CO2RR highly depends on the electrocatalyst. Sn catalysts are considered promising cathode materials for the production of formic acid. The selectivity of Sn catalysts can be regulated by controlling their morphology or alloying them with secondary metals. Herein, we enhanced the selectivity of CO2 reduction to formic acid by synthesizing Sn–Cu@Sn dendrites that have a core@shell architecture. The Sn–Cu@Sn dendrites were prepared by a scalable electro-deposition method. The electronic structure was modified to suppress a reaction pathway for CO production on the Sn surface. Notably, the Sn shell inhibited the cathodic corrosion of Cu during the CO2RR. On a gas diffusion electrode, the Sn–Cu@Sn dendrites exhibited 84.2% faraday efficiency to formic acid for 120 h with high stability.
- This article is part of the themed collection: CO2 capture and conversion


 Please wait while we load your content...
Please wait while we load your content...