Themed collection Most popular 2019-2020 organic chemistry articles

C–H functionalization reactions enabled by hydrogen atom transfer to carbon-centered radicals
Intramolecular and intermolecular HAT to C-centered radicals enables selective C–H functionalization of organic molecules.

Chem. Sci., 2020,11, 12974-12993
https://doi.org/10.1039/D0SC04881J
New avenues for C–B bond formation via radical intermediates
Efficient radical routes to important alkyl and aryl boronic esters have been developed over the past few years. Such reactions are complementary to existing transition-metal catalysed cross coupling processes.
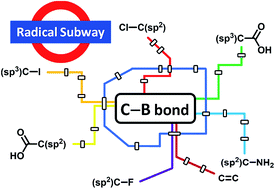
Chem. Sci., 2019,10, 8503-8518
https://doi.org/10.1039/C9SC03765A
A synthetic chemist's guide to electroanalytical tools for studying reaction mechanisms
A range of electroanalytical tools can be applied to studying redox reactions, probing key mechanistic questions in synthetic chemistry.
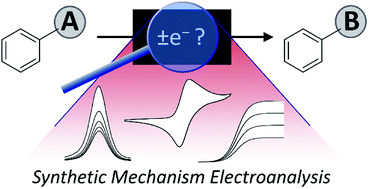
Chem. Sci., 2019,10, 6404-6422
https://doi.org/10.1039/C9SC01545K
Toward ideal carbon dioxide functionalization
From carbon fixation, Grignard reaction, metal-catalyzed reactions and asymmetric CO2-incorporation, what would be the ideal CO2-functionalization?
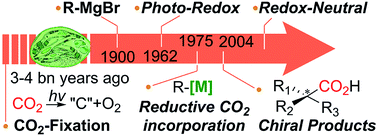
Chem. Sci., 2019,10, 3905-3926
https://doi.org/10.1039/C8SC05539D
Allylic C(sp3)–H alkylation via synergistic organo- and photoredox catalyzed radical addition to imines
A new catalytic method for the direct alkylation of allylic C(sp3)–H bonds from unactivated alkenes via synergistic organo- and photoredox catalysis is described.
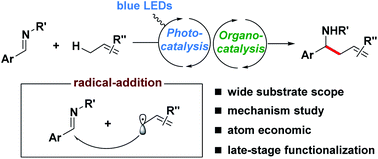
Chem. Sci., 2020,11, 4954-4959
https://doi.org/10.1039/D0SC00819B
N-Heterocyclic carbene-catalyzed deaminative cross-coupling of aldehydes with Katritzky pyridinium salts
By employing an N-heterocyclic carbene (NHC) catalyst, we developed a versatile catalytic system that enables deaminative cross-coupling reactions of aldehydes with redox-active pyridinium salts.
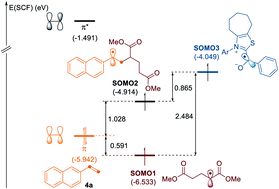
Chem. Sci., 2020,11, 3192-3197
https://doi.org/10.1039/D0SC00225A
Regiodivergent construction of medium-sized heterocycles from vinylethylene carbonates and allylidenemalononitriles
Here we report palladium-catalyzed, regiodivergent [5 + 4] and [5 + 2] annulations of vinylethylene carbonates and allylidenemalononitriles affording over 50 medium-sized heterocycles in high isolated yields with excellent regioselectivities.
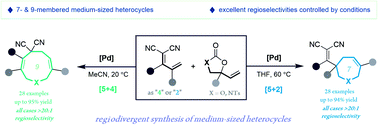
Chem. Sci., 2020,11, 2888-2894
https://doi.org/10.1039/C9SC06377C
Formyl-selective deuteration of aldehydes with D2O via synergistic organic and photoredox catalysis
Formyl-selective deuteration of aldehydes with D2O mediated by the synergistic combination of light-driven, polyoxometalate-facilitated HAT and thiol catalysis is reported.
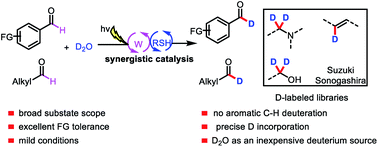
Chem. Sci., 2020,11, 1026-1031
https://doi.org/10.1039/C9SC05132E
Synthesis of amino acids and peptides with bulky side chains via ligand-enabled carboxylate-directed γ-C(sp3)–H arylation
Pd-catalyzed ligand-enabled γ-C(sp3)–H arylation of tert-leucine and its derived peptides without using an external directing group via a less favored six-membered palladacycle is reported.
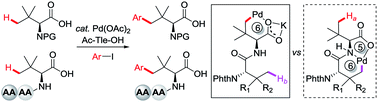
Chem. Sci., 2020,11, 290-294
https://doi.org/10.1039/C9SC04482E
Sulfamides direct radical-mediated chlorination of aliphatic C–H bonds
Amine-anchored sulfamides direct radical-mediated chlorination of aliphatic C–H bonds. The site of C–H abstraction can be modulated by varying the sulfamide nitrogen substituents, a feature that has not been demonstrated with other substrate classes.
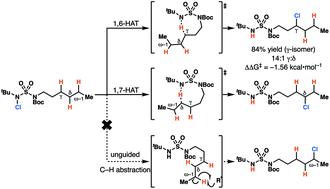
Chem. Sci., 2020,11, 217-223
https://doi.org/10.1039/C9SC03428E
Secondary amine selective Petasis (SASP) bioconjugation
Secondary amine selective Petasis (SASP) bioconjugation for the selective labeling of peptides and proteins with N-terminal secondary amines.
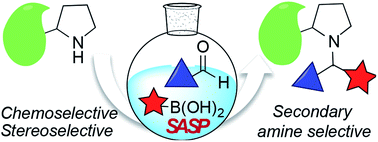
Chem. Sci., 2020,11, 53-61
https://doi.org/10.1039/C9SC04697F
Diastereoselective ring opening of fully-substituted cyclopropanes via intramolecular Friedel–Crafts alkylation
We herein disclose a diastereoselective ring opening of non-donor–acceptor cyclopropanes via an intramolecular Friedel–Crafts alkylation en route to functionalized dihydronaphthalene scaffolds possessing quaternary carbon stereocentres.

Chem. Sci., 2019,10, 9548-9554
https://doi.org/10.1039/C9SC03832A
Oxidant speciation and anionic ligand effects in the gold-catalyzed oxidative coupling of arenes and alkynes
The mechanism of the gold-catalyzed oxidative cross-coupling of arenes and alkynes has been studied in detail combining stoichiometric experiments with putative reaction intermediates and DFT calculations.

Chem. Sci., 2019,10, 8411-8420
https://doi.org/10.1039/C9SC02372K
Solid-state Suzuki–Miyaura cross-coupling reactions: olefin-accelerated C–C coupling using mechanochemistry
The first general solid-state Suzuki–Miyaura cross-coupling reactions using mechanochemistry has been developed.
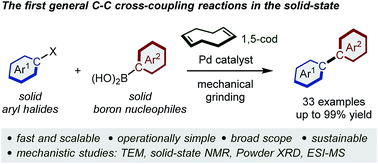
Chem. Sci., 2019,10, 8202-8210
https://doi.org/10.1039/C9SC02185J
A dual photoredox-nickel strategy for remote functionalization via iminyl radicals: radical ring-opening-arylation, -vinylation and -alkylation cascades
A divergent strategy for the remote arylation, vinylation and alkylation of nitriles is described.

Chem. Sci., 2019,10, 7728-7733
https://doi.org/10.1039/C9SC02616A
Electrochemical C–H oxygenation and alcohol dehydrogenation involving Fe-oxo species using water as the oxygen source
The well-known [(TAML)Fe(OH2)]− complex undergoes proton-coupled oxidation to an Fe-oxo species that supports electrochemical C–H oxidation and alcohol dehydrogenation.
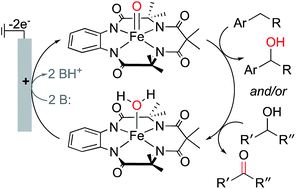
Chem. Sci., 2019,10, 7542-7548
https://doi.org/10.1039/C9SC02609F
Rhodium catalyzed template-assisted distal para-C–H olefination
We report first example of template assisted rhodium catalyzed para-C–H alkenylation.

Chem. Sci., 2019,10, 7426-7432
https://doi.org/10.1039/C9SC01824G
Trifluoromethylthiolation–arylation of diazocarbonyl compounds by modified Hooz multicomponent coupling
Multicomponent reaction of diazocarbonyl and dibenzenesulfonimide-SCF3 reagents with BAr4 salts in the presence of Zn(NTf2)2 gives α,α′-difunctionalized trifluoromethylthio compounds.
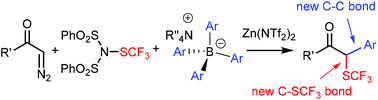
Chem. Sci., 2019,10, 5990-5995
https://doi.org/10.1039/C9SC00829B
Diacetyl as a “traceless” visible light photosensitizer in metal-free cross-dehydrogenative coupling reactions
Minisci alkylation is of prime importance for its applicability in functionalizing diverse heteroarenes, which are core structures in many bioactive compounds.
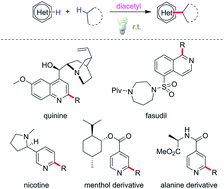
Chem. Sci., 2019,10, 5018-5024
https://doi.org/10.1039/C8SC05631E
Direct conversion of phenols into primary anilines with hydrazine catalyzed by palladium
A general and practical method to directly convert phenols into primary anilines with cheap and easy-to-handle hydrazine as the amine and hydride sources catalyzed by Pd/C.

Chem. Sci., 2019,10, 4775-4781
https://doi.org/10.1039/C9SC00595A
Primary α-tertiary amine synthesis via α-C–H functionalization
A reactive ketimine intermediate was demonstrated to be intercepted by a variety of nucleophiles including organometallics and TMSCN.
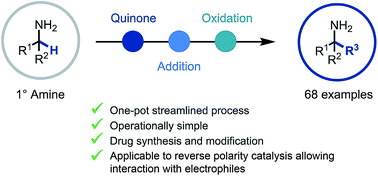
Chem. Sci., 2019,10, 3401-3407
https://doi.org/10.1039/C8SC05164J
Synthesis of 18F-difluoromethylarenes using aryl boronic acids, ethyl bromofluoroacetate and [18F]fluoride
Herein, we report the radiosynthesis of 18F-difluoromethylarenes via the assembly of three components, a boron reagent, ethyl bromofluoroacetate, and cyclotron-produced non-carrier added [18F]fluoride.
![Graphical abstract: Synthesis of 18F-difluoromethylarenes using aryl boronic acids, ethyl bromofluoroacetate and [18F]fluoride](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8SC05096A&imageInfo.ImageIdentifier.Year=2019)
Chem. Sci., 2019,10, 3237-3241
https://doi.org/10.1039/C8SC05096A
Catalytic β C–H amination via an imidate radical relay
An iodine-catalyzed strategy for β C–H amination of alcohols is enabled by a chemo-, regio-, and stereo-selective H-atom transfer mechanism.
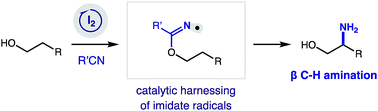
Chem. Sci., 2019,10, 2693-2699
https://doi.org/10.1039/C8SC05685D
Enabling synthesis in fragment-based drug discovery by reactivity mapping: photoredox-mediated cross-dehydrogenative heteroarylation of cyclic amines
A nanogram-to-gram workflow has been established for the identification and development of synthetic transformations which are enabling in Fragment-Based Drug Discovery (FBDD). In this study, we disclose a method for the synthesis of privileged sp2–sp3 architectures via direct cross-dehydrogenative coupling of heterocycles.
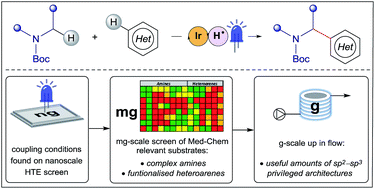
Chem. Sci., 2019,10, 2264-2271
https://doi.org/10.1039/C8SC04789H
A modular approach to prepare enantioenriched cyclobutanes: synthesis of (+)-rumphellaone A
A modular synthesis of enantioenriched polyfunctionalized cyclobutanes was developed and applied to the synthesis of (+)-rumphellaone A.
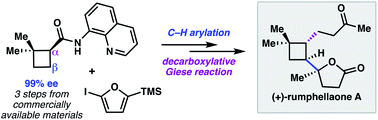
Chem. Sci., 2019,10, 2315-2319
https://doi.org/10.1039/C8SC05444D
Visible-light-mediated Minisci C–H alkylation of heteroarenes with unactivated alkyl halides using O2 as an oxidant
Herein, we report a protocol for direct visible-light-mediated Minisci C–H alkylation of heteroarenes with unactivated alkyl halides using molecular oxygen as an oxidant at room temperature.

Chem. Sci., 2019,10, 976-982
https://doi.org/10.1039/C8SC04892D
δ C–H (hetero)arylation via Cu-catalyzed radical relay
A radical relay strategy has been developed to enable selective δ C–H arylation. The approach employs a chiral copper catalyst, which serves the dual roles of generating an N-centered radical to promote intramolecular H-atom transfer, and then intercepting a distal C-centered radical for C–C bond formation with (hetero)aryl boronic acids.
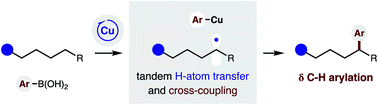
Chem. Sci., 2019,10, 1207-1211
https://doi.org/10.1039/C8SC04366C
Photoredox-mediated remote C(sp3)–H heteroarylation of free alcohols
We report an efficient and economical method for remote δ C(sp3)–H heteroarylation of free aliphatic alcohols using a hypervalent iodine PFBI-OH oxidant under photoredox catalysis.
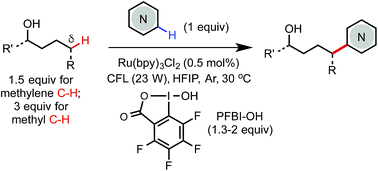
Chem. Sci., 2019,10, 688-693
https://doi.org/10.1039/C8SC04134B
Rhodium(II)-catalyzed C–H aminations using N-mesyloxycarbamates: reaction pathway and by-product formation
DFT study to elucidate the mechanism of Rh-catalyzed C–H aminations with N-mesyloxycarbamates and the pathway by which by-products formed.
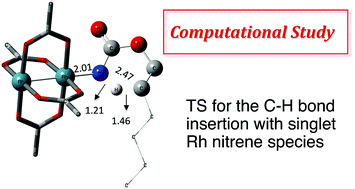
Chem. Sci., 2019,10, 718-729
https://doi.org/10.1039/C8SC03153C
Transition-metal free C–C bond cleavage/borylation of cycloketone oxime esters
An efficient transition-metal free C–C bond cleavage/borylation of cycloketone oxime esters has been described. In this reaction, the B2(OH)4 reagent not only served as the boron source but also acted as an electron donor source through formation of a complex with a DMAc-like Lewis base.

Chem. Sci., 2019,10, 161-166
https://doi.org/10.1039/C8SC03315C
About this collection
This specially curated collection pulls together some of the most popular articles from 2019 and 2020 in the field of organic chemistry. The collection presents some outstanding contributions to the field, ranging from electroanalytical tools for studying reaction mechanisms through to C-H functionalisation methodology, and as with all Chemical Science articles – they are all completely free to access and read. We hope you enjoy browsing through this collection.
See also:
Most popular 2019-2020 inorganic, main group and crystal engineering chemistry articles
Most popular 2019-2020 materials and energy chemistry articles
Most popular 2019-2020 physical and theoretical chemistry articles
Most popular 2019-2020 catalysis articles
Most popular 2019-2020 analytical chemistry articles
Most popular 2019-2020 supramolecular chemistry articles
Most popular 2019-2020 chemical biology articles
Most popular 2019-2020 review articles