Regiodivergent construction of medium-sized heterocycles from vinylethylene carbonates and allylidenemalononitriles†
Abstract
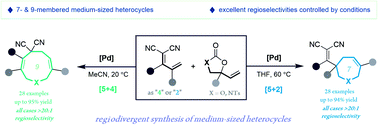
Medium-sized heterocycles exist in a broad spectrum of biologically active natural products and medicinally important synthetic compounds. The construction of medium-sized rings remains challenging, particularly the assembly of different ring sizes from the same type of substrate. Here we report palladium-catalyzed, regiodivergent [5 + 4] and [5 + 2] annulations of vinylethylene carbonates and allylidenemalononitriles. We describe the production of over 50 examples of nine- and seven-membered heterocycles in high isolated yields and excellent regioselectivities. We demonstrate the synthetic utility of this approach by converting a nine-membered ring product to an interesting polycyclic caged molecule via a [2 + 2] transannulation. Mechanistic studies suggest that the [5 + 2] annulation proceeds through palladium-catalyzed ring-opening/re-cyclization from the [5 + 4] adducts.
- This article is part of the themed collections: Most popular 2019-2020 organic chemistry articles and In celebration of Chinese New Year


 Please wait while we load your content...
Please wait while we load your content...