New avenues for C–B bond formation via radical intermediates
Abstract
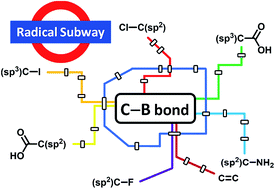
This perspective gives an overview on recent findings in the emerging area of C-radical borylation using diborons as radical trapping reagents. Aryl, vinyl and alkyl boronic esters can be accessed via such an approach under mild conditions. These processes are complementary to established transition metal catalysed cross coupling reactions. Radical borylations can be conducted in the absence of a transition metal but some processes require transition metals as catalysts. It will be shown that various readily available C-radical precursors can be used to run these borylations. For a better understanding of the chemistry, mechanistic discussions are also presented and an outlook on this topic will be provided at the end of the article.
- This article is part of the themed collections: Most popular 2019-2020 organic chemistry articles, Most popular 2018-2019 organic chemistry articles, Most popular 2018-2019 review articles and 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...