N-Heterocyclic carbene-catalyzed deaminative cross-coupling of aldehydes with Katritzky pyridinium salts†
Abstract
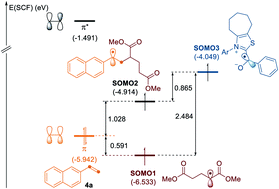
By employing an N-heterocyclic carbene (NHC) catalyst, we developed a versatile catalytic system that enables deaminative cross-coupling reactions of aldehydes with redox-active pyridinium salts. Katritzky pyridinium salts behave as single-electron oxidants capable of generating alkyl radicals enabled by the redox properties of the enolate form of Breslow intermediates. The resultant alkyl radical undergoes efficient recombination with the NHC-bound aldehyde-derived carbonyl carbon radical for the formation of a C–C bond. The mild and transition metal-free reaction conditions tolerate a broad range of functional groups, and its utility has been further demonstrated by the modification of a series of peptide feedstocks and application to the three-component dicarbofunctionalization of olefins.
- This article is part of the themed collections: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS), Most popular 2019-2020 organic chemistry articles and Celebrating Chemical Science in Korea


 Please wait while we load your content...
Please wait while we load your content...