Synthesis of amino acids and peptides with bulky side chains via ligand-enabled carboxylate-directed γ-C(sp3)–H arylation†
Abstract
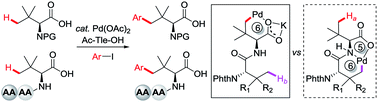
Amino acids and peptides with bulky side chains are of significant importance in organic synthesis and modern medicinal chemistry. The efficient synthesis of these molecules with full enantiocontrol and high diversity remains challenging. Herein we report a Pd-catalyzed ligand-enabled γ-C(sp3)–H arylation of tert-leucine and its derived peptides without using an external directing group (DG) via a less favored six-membered palladacycle. Structurally diverse bulky side chain amino acids and peptides were accessed in a step-economic fashion and the reaction could be conducted on a gram scale with retention of chirality. The resulting amino acids can be used as chiral ligands in Co(III)-catalyzed enantioselective C(sp3)–H amidation. It is worth noting that the weakly coordinating carboxylate DG outcompetes the strongly coordinating bidentate DG of the peptide backbone, providing the products of γ-C(sp3)–H arylation of Tle residue exclusively. This protocol represents the first example of late stage C(sp3)–H functionalization of peptides using a weakly coordinating directing group.
- This article is part of the themed collections: Most popular 2019-2020 organic chemistry articles and In celebration of Chinese New Year


 Please wait while we load your content...
Please wait while we load your content...