A modular approach to prepare enantioenriched cyclobutanes: synthesis of (+)-rumphellaone A†
Abstract
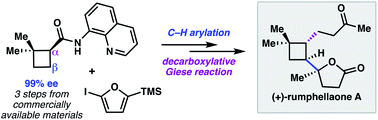
A modular synthesis of enantioenriched polyfunctionalized cyclobutanes was developed that features an 8-aminoquinolinamide directed C–H arylation reaction. The C–H arylation products were derivatized through subsequent decarboxylative coupling processes. This synthetic strategy enabled a 9-step enantioselective total synthesis of the antiproliferative meroterpenoid (+)-rumphellaone A.
- This article is part of the themed collections: Most popular 2019-2020 organic chemistry articles, New reactivity in organic chemistry and Editor's choice - Paolo Melchiorre


 Please wait while we load your content...
Please wait while we load your content...