Themed collection HOT articles in Organic Chemistry Frontiers for 2014

Asymmetric cobalt catalysts for hydroboration of 1,1-disubstituted alkenes
Chiral iminopyridine oxazoline (IPO) ligands were designed, synthesized and utilized for the first cobalt-catalyzed highly regio- and enantioselective anti-Markovnikov hydroboration of 1,1-disubstituted aryl alkenes. These novel IPO ligands will likely be of high value for asymmetric transformations with first-row transition metals.
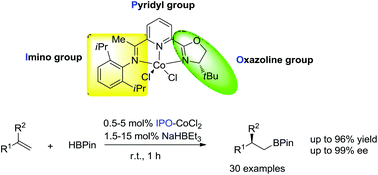
Org. Chem. Front., 2014,1, 1306-1309
https://doi.org/10.1039/C4QO00295D
Formal fluorine atom transfer radical addition: silver-catalyzed carbofluorination of unactivated alkenes with ketones in aqueous solution
Using a combination of ketones and Selectfluor to mimic α-fluoroketones, formal fluorine-atom-transfer radical addition (F-ATRA) reactions were successfully developed in high efficiency.
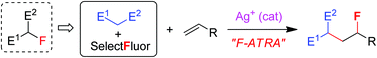
Org. Chem. Front., 2014,1, 1299-1305
https://doi.org/10.1039/C4QO00256C
Efficient access to 1H-indazoles via copper-catalyzed cross-coupling/cyclization of 2-bromoaryl oxime acetates and amines
A novel synthesis of 1H-indazoles via copper-catalyzed cross-coupling/cyclization of 2-bromoaryl oxime acetates and amines is reported.
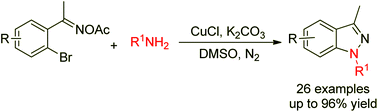
Org. Chem. Front., 2014,1, 1295-1298
https://doi.org/10.1039/C4QO00244J
Efficient and scalable Pd-catalyzed double aminocarbonylations under atmospheric pressure at low catalyst loadings
A scalable approach to access various pharmaceutical and structurally intriguing N-substituted phthalimides via double aminocarbonylations has been established under atmospheric CO pressure with a robust Pd-NHC complex at a low catalyst loading.
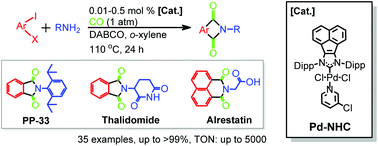
Org. Chem. Front., 2014,1, 1261-1265
https://doi.org/10.1039/C4QO00253A
Function through bio-inspired, synthesis-informed design: step-economical syntheses of designed kinase inhibitors
We describe here step-economical, function-oriented strategies towards the syntheses of potent kinase inhibitors inspired by the natural product staurosporine.
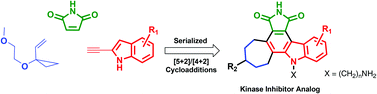
Org. Chem. Front., 2014,1, 1166-1171
https://doi.org/10.1039/C4QO00228H
Acenaphthoimidazolium chloride-enabled nickel-catalyzed amination of bulky aryl tosylates
A robust catalyst derived from NiCl2·(DME) and acenaphthoimidazolium chloride exhibited high activity towards the challenging amination of bulky and heterocyclic aryl tosylates with secondary and primary amines under mild reaction conditions.
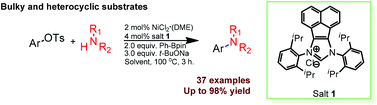
Org. Chem. Front., 2014,1, 1172-1175
https://doi.org/10.1039/C4QO00233D
A sidearm-assisted phosphine for catalytic ylide intramolecular cyclopropanation
The first phosphine-catalyzed cyclopropanation reaction via covalent ylide catalysis has been realized with high efficiency in the presence of sidearm-assisted phosphine catalysts.
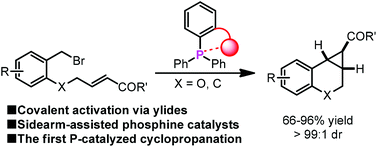
Org. Chem. Front., 2014,1, 1035-1039
https://doi.org/10.1039/C4QO00232F
Facile solid-phase synthesis of PNA–peptide conjugates using pNZ-protected PNA monomers
A p-nitrobenzyloxycarbonyl/bis-Boc strategy was developed for efficient synthesis of versatile PNA–peptide conjugates.
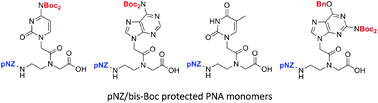
Org. Chem. Front., 2014,1, 1050-1054
https://doi.org/10.1039/C4QO00217B
Generation of 1-amino-isoquinoline-N-oxides via a tandem reaction of 2-alkynylbenzaldoxime with secondary amines in the presence of silver(I) and copper(I)
1-Amino-isoquinoline-N-oxides are generated under mild conditions in good yields through a silver(I) and copper(I) catalyzed reaction of 2-alkynylbenzaldoxime with secondary amines.
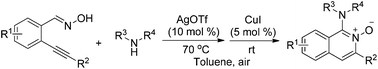
Org. Chem. Front., 2014,1, 1045-1049
https://doi.org/10.1039/C4QO00209A
Two- and three-coordinate formal iron(I) compounds featuring monodentate aminocarbene ligands
Monodentate aminocarbene ligands, both NHC and cAAC, are capable of stabilizing low-coordinate iron(I) compounds.

Org. Chem. Front., 2014,1, 1040-1044
https://doi.org/10.1039/C4QO00175C
Near-infrared absorbing heterocyclic quinoid donors for organic solar cell devices
Near-infrared absorbing heterocyclic quinoid molecules have been developed as donor materials for organic photovoltaic devices.

Org. Chem. Front., 2014,1, 988-991
https://doi.org/10.1039/C4QO00182F
Enyne [4 + 4] photocycloaddition with polycyclic aromatics
Polycyclic aromatics undergo [4 + 4] photocycloaddition to give tricyclic polyene products.
![Graphical abstract: Enyne [4 + 4] photocycloaddition with polycyclic aromatics](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4QO00190G&imageInfo.ImageIdentifier.Year=2014)
Org. Chem. Front., 2014,1, 961-964
https://doi.org/10.1039/C4QO00190G
Highly efficient and stereocontrolled oxidative coupling of tetrahydropyrroloindoles: synthesis of chimonanthines, (+)-WIN 64821 and (+)-WIN 64745
The I2-mediated oxidative dimerization of tetrahydropyrroloindoles allows the convenient and efficient synthesis of target molecules.
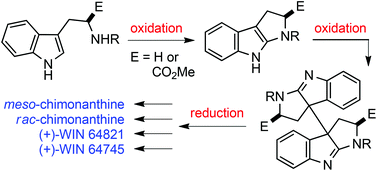
Org. Chem. Front., 2014,1, 956-960
https://doi.org/10.1039/C4QO00165F
Stereocontrolled synthesis of propionate motifs from L-lactic and L-alanine aldehydes. A DFT study of the hydrogen transfer under endocyclic control
A sequence of Mukaiyama aldol and radical hydrogen transfer reactions was applied to α-alkoxy and α-aminoaldehydes for the synthesis of propionate motifs and subunits thereof.
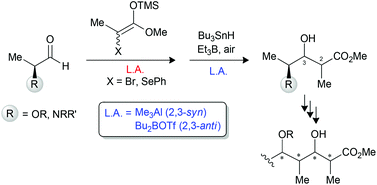
Org. Chem. Front., 2014,1, 974-982
https://doi.org/10.1039/C4QO00142G
Stereospecific synthesis of highly functionalized benzo[3.1.0]bicycloalkanes via multistep cascade reactions
A facile approach for the synthesis of benzo[3.1.0]bicycloalkanes via alkylation/cyclopropanation cascade reactions of benzyl bromide with triphenylphosphonium bromide.
![Graphical abstract: Stereospecific synthesis of highly functionalized benzo[3.1.0]bicycloalkanes via multistep cascade reactions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4QO00134F&imageInfo.ImageIdentifier.Year=2014)
Org. Chem. Front., 2014,1, 965-968
https://doi.org/10.1039/C4QO00134F
Copper-mediated difluoromethylation of electron-poor aryl iodides at room temperature
The direct difluoromethylation of electron-poor aryl iodides, as well as heteroaryl and β-styryl iodides, using TMSCF2H at ambient temperature was developed.
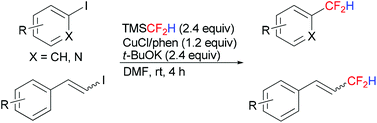
Org. Chem. Front., 2014,1, 774-776
https://doi.org/10.1039/C4QO00153B
Design of a new series of chiral phosphite–olefin ligands and their application in asymmetric catalysis
A novel type of open-chain chiral chelating olefin bearing a simple monophosphite unit with a binaphthyl backbone was designed and synthesized for asymmetric catalysis.
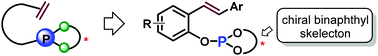
Org. Chem. Front., 2014,1, 738-741
https://doi.org/10.1039/C4QO00135D
A problem solving approach for the diastereoselective synthesis of (5′S)- and (5′R)-5′,8-cyclopurine lesions
A high-yielding approach for the diastereoselective syntheses of the four (5′S)- and (5′R)-5′,8-cyclopurine lesions and their phosphoramidites has been developed.
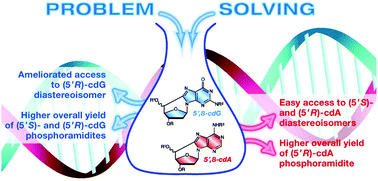
Org. Chem. Front., 2014,1, 698-702
https://doi.org/10.1039/C4QO00133H
A green fullerene derivative as a fluoride ion sensor
The fluoride ion detection limit is at the micromolar level.
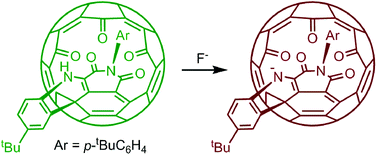
Org. Chem. Front., 2014,1, 652-656
https://doi.org/10.1039/C4QO00111G
Selective monofluorination of active methylene compounds: the important role of ZnCl2 in inhibiting overfluorination
ZnCl2 effectively facilitates selective monofluorination of methylene compounds by inhibiting the undesired overfluorination.
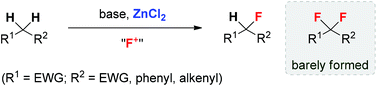
Org. Chem. Front., 2014,1, 625-629
https://doi.org/10.1039/C4QO00090K
Palladium-catalyzed reductive cleavage of tosylated arenes using isopropanol as the mild reducing agent
The reduction (deuteration) of oxygenated arenes can be achieved by Pd/CM-phos catalyst system via a tosylation pathway.
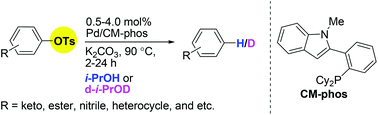
Org. Chem. Front., 2014,1, 464-467
https://doi.org/10.1039/C4QO00103F
Trienamine catalysis with linear deconjugated 3,5-dienones
Deconjugated linear 3,5-dienones with substantial substitutions were used in β,ε-regioselective Diels–Alder cycloadditions with 3-olefinic oxindoles via trienamine catalysis of cinchona-based primary amines.
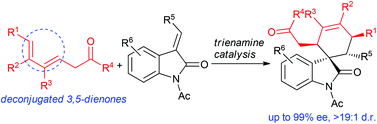
Org. Chem. Front., 2014,1, 490-493
https://doi.org/10.1039/C4QO00079J
Vanadium-catalyzed C(sp3)–H fluorination reactions
Vanadium(III) oxide catalyzes the direct fluorination of C(sp3)–H groups with Selectfluor.

Org. Chem. Front., 2014,1, 468-472
https://doi.org/10.1039/C4QO00057A
Singlet carbenes as mimics for transition metals: synthesis of an air stable organic mixed valence compound [M2(C2)+˙; M = cyclic(alkyl)(amino)carbene]
A bis-carbene-stabilized ethynyl radical cation, a purely organic mixed valence compound, is indefinitely air stable.
![Graphical abstract: Singlet carbenes as mimics for transition metals: synthesis of an air stable organic mixed valence compound [M2(C2)+˙; M = cyclic(alkyl)(amino)carbene]](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4QO00072B&imageInfo.ImageIdentifier.Year=2014)
Org. Chem. Front., 2014,1, 351-354
https://doi.org/10.1039/C4QO00072B
A concise method to prepare novel fused heteroaromatic diones through double Friedel–Crafts acylation
Different types of fused heteroaromatic diones have been successfully prepared through the concise intramolecular double Friedel–Crafts acylation strategy.
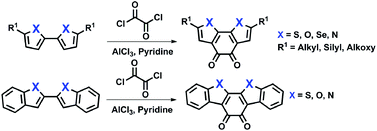
Org. Chem. Front., 2014,1, 391-394
https://doi.org/10.1039/C4QO00032C
Palladium-catalysed regioselective hydroamination of 1,3-dienes: synthesis of allylic amines
The combination of Pd(cod)Cl2 and L9 allows for the selective 1,4-hydroamination of a series of acyclic and cyclic dienes with a variety of aromatic and aliphatic amines under relatively mild conditions. The reaction gives exclusive formation of synthetically useful allylic amines with high regio- and chemoselectivity and does not need any additives.
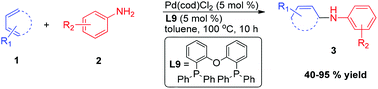
Org. Chem. Front., 2014,1, 368-372
https://doi.org/10.1039/C4QO00023D
Sterically demanding aryl–alkyl Suzuki–Miyaura coupling
Efficient sterically demanding aryl–alkyl Suzuki–Miyaura coupling between di-ortho-substituted aryl halides and secondary alkylboronic acids has been achieved with a Pd-AntPhos catalyst.
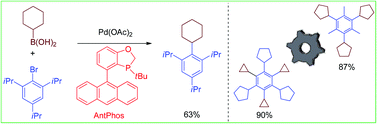
Org. Chem. Front., 2014,1, 225-229
https://doi.org/10.1039/C4QO00024B
Synthesis of β-methyl-α-methylene-γ-butyrolactone from biorenewable itaconic acid
A high-yielding route to β-methyl-α-methylene-γ-butyrolactone, a monomer for the production of high-performance engineering bioplastics, from biorenewable and inexpensive itaconic acid has been developed.
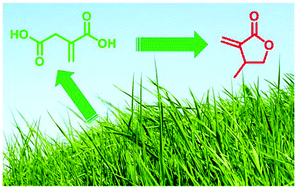
Org. Chem. Front., 2014,1, 230-234
https://doi.org/10.1039/C3QO00089C
Copper-catalyzed three-component synthesis of 5-acetamidoimidazoles from carbodiimides, acyl chlorides and isocyanides
Three-component reaction of carbodiimides, acyl chlorides, and isocyanides took place smoothly at room temperature to afford 1,4-disubstituted 5-acetamidoimidazoles in good yields.
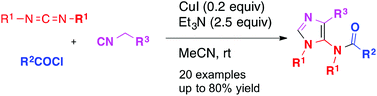
Org. Chem. Front., 2014,1, 240-246
https://doi.org/10.1039/C4QO00034J
Highly selective 4-alkynoic acids synthesis via iron-mediated complete inversion of stereogenic carbon centers
An Fe-catalyzed highly regio- and diastereoselective reaction of 4-ethynyloxetan-2-ones with different Grignard reagents affording alkynes 3 in good yields with absolute configuration of the β-carbon atom completely inverted has been developed.
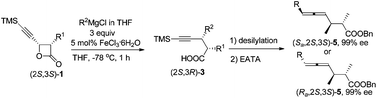
Org. Chem. Front., 2014,1, 247-252
https://doi.org/10.1039/C3QO00066D
Solvent-driven selective π-cation templating in dynamic assembly of interlocked molecules
The different solvent responses for bipyridinium and trispyridinium-based dynamic imine [2]rotaxanes allow their interconversion with high selectivity.
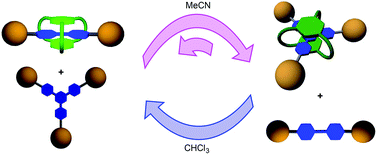
Org. Chem. Front., 2014,1, 167-175
https://doi.org/10.1039/C3QO00074E
Highly diasteroselective intermolecular 1,3-dipolar cycloaddition reactions of carbonyl ylides with aldimines to afford sterically disfavored cis-oxazolidines
Sterically disfavored oxazolidines were obtained via a Rh(II)/Ag(I)-catalyzed [3 + 2] exo-cycloaddition reaction. Subsequent hydrolysis gave trans-β-amino-α-hydroxyl ester derivatives in high yields.

Org. Chem. Front., 2014,1, 181-185
https://doi.org/10.1039/C3QO00040K
Palladium-catalyzed base-accelerated direct C–H bond alkenylation of phenols to synthesize coumarin derivatives
A palladium-catalyzed base-accelerated ortho-selective C–H alkenylation of phenols to synthesize bioactive coumarin derivatives was developed featuring a wide substrate scope under mild reaction conditions. Several bioactive molecules were functionalized and several coumarins with bioactive properties were synthesized.
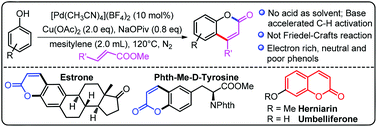
Org. Chem. Front., 2014,1, 44-49
https://doi.org/10.1039/C3QO00010A
Transition-metal-free, room-temperature radical azidofluorination of unactivated alkenes in aqueous solution
Transition-metal-free radical azidofluorination of unactivated alkenes was successfully achieved in aqueous solution at room temperature with excellent regioselectivity and functional group compatibility.

Org. Chem. Front., 2014,1, 100-104
https://doi.org/10.1039/C3QO00037K
About this collection
Read some of the best research published by Organic Chemistry Frontiers in 2014. These articles are recommended by OCF referees as being of significant novelty and interest.