Asymmetric cobalt catalysts for hydroboration of 1,1-disubstituted alkenes†
Jianhui
Chen
,
Tuo
Xi
,
Xiang
Ren
,
Biao
Cheng
,
Jun
Guo
and
Zhan
Lu
*
Department of Chemistry, Zhejiang University, 148 Tianmushan Road Hangzhou, Zhejiang, 310028, China. E-mail: luzhan@zju.edu.cn
First published on 19th November 2014
Abstract
Chiral iminopyridine oxazoline (IPO) ligands were designed, synthesized and utilized for the first cobalt-catalyzed highly regio- and enantioselective anti-Markovnikov hydroboration of 1,1-disubstituted aryl alkenes. These novel IPO ligands will likely be of high value for asymmetric transformations with first-row transition metals.
Introduction
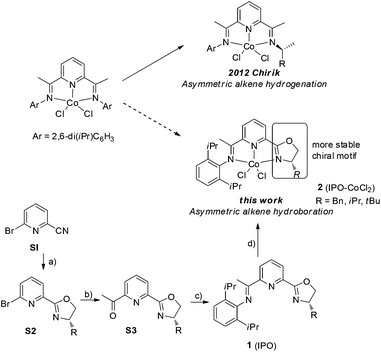
Asymmetric hydroboration of alkenes is one of the most useful methods to form chiral alkylboronic acid derivatives, which are widely used in organic synthesis.1 Hydroboration of terminal alkenes catalyzed by chiral transition metals favors Markovinov regioselectivity.2 Catalytic asymmetric anti-Markovnikov hydroboration of 1,1-disubstituted alkenes remains a challenge.3 Low enantioselectivity and in some cases poor regioselectivity were obtained through Rh- and Ir-catalyzed reactions of 1,1-disubstituted alkenes with catecholborane.4 Recently, two catalytic systems, Iridium with chiral PN-ligand5 and copper with chiral NHC ligand,6 were reported which realized asymmetric hydroboration of 1,1-disubstituted alkenes. However, a noble transition metal was used, or B2(pin)2 was used which provides more waste. A few cases showed high enantioselectivity (≥90% ee). To the best of our knowledge,7 there is no previous report on asymmetric cobalt-catalyzed anti-Markovnikov hydroboration of 1,1-disubtituted alkenes.8Noble metals play a very important role in asymmetric organic transformations in academia and industry, such as in the asymmetric hydrogenation of alkenes,9 however, earth-abundant metal catalysts often react via one-electron processes, which are limited to certain type of reactions. Redox-active ligands, which have been studied for their spectroscopic properties, might provide the possibility for earth-abundant metals to go through two-electron redox processes to promote bond-breaking and making events.10 The asymmetric applications of redox-active ligands are extremely rare. Recently, the Chirik group reported a highly enantioselective hydrogenation of alkenes11 using C1-symmetric bis(imino)pyridine cobalt complexes,12 which shows that the use of a chiral redox-active ligand gives a potential good class of catalysts for asymmetric organic synthesis, however, the chiral imine on the catalyst is not stable and is easily removed. Based on bi(imino)pyridine ligands, we introduced chiral oxazoline units as stereodirecting element13 and designed the new iminopyridine oxazoline (IPO) cobalt complexes, in which the iminopyridine group is proposed to stabilize the cobalt and chiral oxazoline group to control the enantioselectivity (Scheme 1).
Herein we report the synthesis of a series of chiral IPO ligands from commercially available starting materials (Scheme 1). The cobalt complexes 2 could be synthesized by combining cobalt dichloride with the corresponding ligands and are bench-stable.
Results and discussion
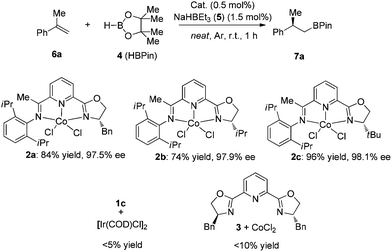
We chose the hydroboration of styrene 6a with HBpin as our model reaction to test the reactivity of our designed chiral cobalt complexes (IPO-CoCl2). Using only 0.5 mol% cobalt complex and 1.5 mol% of NaHBEt3 (1 M in THF solution) as the reductant without any additive solvent, high reactivity and regio- and enantioselectivities were observed in all cases among which complex 2c gave the best yield and highest enantioselectivity (Scheme 2). The reaction was really slow using 1c with iridium catalyst, which might illustrate that IPO ligands work better with first-row transition metals than with late-transition metals. Poor reactivities were achieved when using the bisoxazoline ligand instead of IPO ligands which led us to propose that the iminopyridine group is stabilizing the cobalt.With the best complex (2c) in hand, studies exploring the scope of this process are summarized in Chart 1. The reactions were operated using a Schlenk line at 2.5 mmol scale, not necessarily in a glove box. (1) The reaction showed high enantioselectivities with a variety of substituted α-methyl styrenes, including both electron-rich and electron-deficient styrene compounds; (2) Halides and protected heteroatoms can be tolerated at the para, meta and ortho-positions on the aryl rings; (3) Although slightly low ee’s were observed in the reaction of ortho-substituted styrenes, high yields were obtained; (4) Long alkyl chains at the α-position of styrenes, even with functionalized alkyl chains, were tolerated to prepare the corresponding hydroboration products with high enantioselectivities; (5) A cyclic styrene with terminal alkene was also reactive to afford 7ab in a slightly lower yield with 95% ee; (6) Gratifyingly, 1,1-dialkyl substituted alkenes 6ac and 6ad also participated in this reaction to give the desired hydroboration products in 72% yield with 33% ee and 70% ee, respectively.
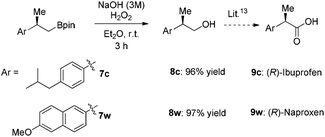
The compounds 7c and 7w can be easily oxidized and further derivatized14 to (R)-naproxen and (R)-ibuprofen, which are well-known non-steroial anti-inflammatory and analgesic drugs15 (Scheme 3).
Conclusions
In conclusion, we have developed a novel iminopyridine oxazoline cobalt-catalyzed highly enantioselective and regioselective anti-Markovnikov hydroboration of 1,1-disubstituted alkenes with hydroborate. A series of useful highly enantiopure borate compounds were easily synthesized from the simple alkenes without any directing group. Current efforts in our lab are underway to explore the applications of IPO ligands in asymmetric reactions.Acknowledgements
Financial support was provided by the “Thousand Youth Talents Plan”, the Fundamental Research Funds for the Central Universities (2013QNA3022), the Starting Funds from Zhejiang University.Notes and references
- For some selected reviews, see: (a) H. C. Brown and B. Singaram, Acc. Chem. Res., 1988, 21, 287 CrossRef CAS; (b) M. Zaidlewicz, in Comprehensive Organometallic Chemistry, ed. G. Wilkinson, F. G. A. Stone and E. W. Abel, Pergamon, Oxford, 1982, vol. 7, p. 229, and references cited therein. For some selected examples, see: Search PubMed; (c) H. C. Brown and G. Zweifel, J. Am. Chem. Soc., 1961, 83, 486 Search PubMed; (d) A. Z. Gonzalez, J. G. Román, E. Gonzalez, J. Martinez, J. R. Medina, K. Matos and J. A. Soderquist, J. Am. Chem. Soc., 2008, 130, 9218 CrossRef CAS PubMed.
- (a) T. Hayashi, Y. Matsumoto and Y. Ito, J. Am. Chem. Soc., 1989, 111, 3426 CrossRef CAS; (b) K. Burgess and M. J. Ohlmeyer, Chem. Rev., 1991, 91, 1179 CrossRef CAS; (c) I. Beletskaya and A. Pelter, Tetrahedron, 1997, 53, 4957 CrossRef CAS; (d) H. Doucet, E. Fernandez, T. P. Layzell and J. M. Brown, Chem. – Eur. J., 1999, 5, 1320 CrossRef CAS; (e) C. M. Crudden and D. Edwards, Eur. J. Org. Chem., 2003, 4695 CrossRef CAS; (f) C. M. Crudden, Y. B. Hleba and A. C. Chen, J. Am. Chem. Soc., 2004, 126, 9200 CrossRef CAS PubMed; (g) S. A. Moteki, D. Wu, K. L. Chandra, D. S. Reddy and J. M. Takacs, Org. Lett., 2006, 8, 3097 CrossRef CAS PubMed; (h) S. A. Moteki and J. M. Takacs, Angew. Chem., Int. Ed., 2008, 47, 894 CrossRef CAS PubMed; (i) C. M. Crudden, B. W. Glasspoole and C. J. Lata, Chem. Commun., 2009, 6704 RSC; (j) S. A. Moteki, K. Toyama, Z. Liu, J. Ma, A. E. Holmes and J. M. Takacs, Chem. Commun., 2012, 263 RSC.
- (a) S. P. Thomas and V. K. Aggarwal, Angew. Chem., Int. Ed., 2009, 48, 1896 CrossRef CAS PubMed. For cobalt-catalyzed racemic hydroboration of alkenes, see: (b) M. Zaidlewicz and J. Meller, Tetrahedron Lett., 1997, 38, 7279 CrossRef CAS; (c) J. V. Obligacion and P. J. Chirik, J. Am. Chem. Soc., 2013, 135, 19107 CrossRef CAS PubMed; (d) L. Zhang, Z. Zuo, X. Leng and Z. Huang, Angew. Chem., Int. Ed., 2014, 53, 2696 CrossRef CAS PubMed. For iron-catalyzed racemic hydroboration of alkenes, see: (e) J. Y. Wu, B. Moreau and T. Ritter, J. Am. Chem. Soc., 2009, 131, 12915 CrossRef CAS PubMed; (f) J. V. Obligacion and P. J. Chirik, Org. Lett., 2013, 15, 2680 CrossRef CAS PubMed; (g) L. Zhang, D. Peng, X. Leng and Z. Huang, Angew. Chem., Int. Ed., 2013, 52, 3676 CrossRef CAS PubMed; (h) M. D. Greenhalgh and S. P. Thomas, Chem. Commun., 2013, 11230 RSC; (i) S. C. Bart, E. Lobkovsky and P. J. Chirik, J. Am. Chem. Soc., 2004, 126, 13794 CrossRef CAS PubMed.
- T. Hayashi and Y. Matsumoto, Tetrahedron: Asymmetry, 1991, 2, 601 CrossRef CAS.
- C. Mazet and D. Gérard, Chem. Commun., 2011, 298 RSC.
- R. Corberán, N. W. Mszar and A. H. Hoveyda, Angew. Chem., Int. Ed., 2011, 50, 7079 CrossRef PubMed.
- For the review on enantioselective cobalt-catalyzed transformations: H. Pellissier and H. Clavier, Chem. Rev., 2014, 114, 2775 CrossRef CAS PubMed.
- Unfortunately, when submitting the manuscript on our independent work a similar result was reported by Z. Huang, L. Zhang, Z.-Q. Zuo, X.-L. Wan and Z. Huang, J. Am. Chem. Soc., 2014, 136, 15501 CrossRef PubMed . However, we used a different strategy for the synthesis of chiral iminopyridine oxazoline since November 14th, 2013.
- For some reviews on asymmetric hydrogenation, see: (a) R. Noyori, Angew. Chem., Int. Ed., 2002, 41, 2008 CrossRef CAS; (b) W. S. Knowles, Angew. Chem., Int. Ed., 2002, 41, 1998 CrossRef CAS; (c) A. J. Minnaard, B. L. Feringa, L. Lefort and J. G. de Vries, Acc. Chem. Res., 2007, 40, 1267 CrossRef CAS PubMed; (d) N. B. Johnson, I. C. Lennon, P. H. Moran and J. A. Ramsden, Acc. Chem. Res., 2007, 40, 1291 CrossRef CAS PubMed; (e) C. S. Schultz and S. W. Krska, Acc. Chem. Res., 2007, 40, 1320 CrossRef PubMed; (f) S. J. Roseblade and A. Pfaltz, Acc. Chem. Res., 2007, 40, 1402 CrossRef CAS PubMed.
- P. J. Chirik and K. Wieghardt, Science, 2010, 327, 794 CrossRef CAS PubMed.
- (a) S. Monfette, Z. R. Turner, S. P. Semproni and P. J. Chirik, J. Am. Chem. Soc., 2012, 134, 4561 CrossRef CAS PubMed; (b) C. Bianchini, G. Mantovani, A. Meli, F. Migliacci, F. Zanobini, F. Laschi and A. Sommazzi, Eur. J. Inorg. Chem., 2003, 1620 CrossRef CAS.
- For the review on bis(imino)pyridines, see: (a) V. C. Gibson, C. Redshaw and G. A. Solan, Chem. Rev., 2007, 107, 1745 CrossRef CAS PubMed. For selected bis(imino)pyridine cobalt-catalyzed examples, see: (b) Q. Knijnenburg, A. D. Horton, H. V. D. Heijden, T. M. Kooistra, D. G. H. Hetterscheid, J. M. M. Smits, B. de Bruin, P. H. M. Budzelaar and A. W. Gal, J. Mol. Catal. A: Chem., 2005, 232, 151 CrossRef CAS PubMed; (c) Q. Knijnenburg, D. Hetterscheid, T. M. Kooistra and P. H. M. Budzelaar, Eur. J. Inorg. Chem., 2004, 1204 CrossRef CAS.
- For some selected reviews on chiral oxazoline ligands, see: (a) P. Braunstein and F. Naud, Angew. Chem., Int. Ed., 2001, 40, 680 CrossRef CAS; (b) H. A. McManus and P. J. Guiry, Chem. Rev., 2004, 104, 4151 CrossRef CAS PubMed; (c) G. C. Hargaden and P. J. Guiry, Chem. Rev., 2009, 109, 2505 CrossRef CAS PubMed; (d) G. Desimoni, G. Faita and K. A. Jørgensen, Chem. Rev., 2011, 111, PR284 CrossRef CAS PubMed.
- P. Galletti, M. Pori and D. Giacomini, Synlett, 2010, 2644 CAS.
- (a) I. T. Harrison, B. Lewis, P. Nelson, W. Rooks, A. Roszkowski, A. Tomolonis and J. H. Fried, J. Med. Chem., 1970, 13, 203 CrossRef CAS; (b) T. Y. Shen, Angew. Chem., Int. Ed. Engl., 1972, 11, 460 CrossRef CAS PubMed; (c) D. Lednicer and L. A. Mitscher, The Organic Chemistry of Drug Synthesis, Wiley, New York, 1977, vol. 1 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4qo00295d |
| This journal is © the Partner Organisations 2014 |

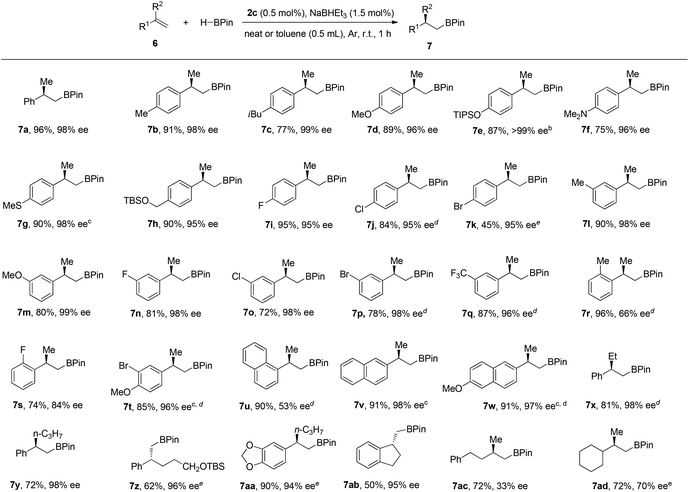
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Standard conditions: unless otherwise noted, 6 (2.5 mmol), HBPin (2.5 mmol), 2c (0.5 mol%), NaBHEt3 (1.5 mol%) at room temperature for 1 h;
Standard conditions: unless otherwise noted, 6 (2.5 mmol), HBPin (2.5 mmol), 2c (0.5 mol%), NaBHEt3 (1.5 mol%) at room temperature for 1 h;