Stereospecific synthesis of highly functionalized benzo[3.1.0]bicycloalkanes via multistep cascade reactions†
Jian-Bo
Zhu
,
Hao
Chen
,
Lijia
Wang
and
Yong
Tang
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China. E-mail: tangy@mail.sioc.ac.cn
First published on 17th June 2014
Abstract
A facile approach for the synthesis of benzo[3.1.0]bicycloalkanes via alkylation/cyclopropanation cascade reactions of benzyl bromide with triphenylphosphonium bromide has been developed. By elaborate designing of starting materials, this multistep cyclization proceeded smoothly with a wide range of substrates, providing the benzo[3.1.0]bicycloalkanes in 52–90% yields with exclusive diastereoselectivity.
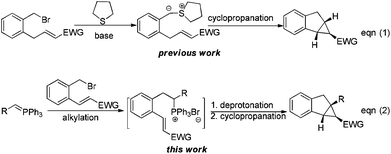
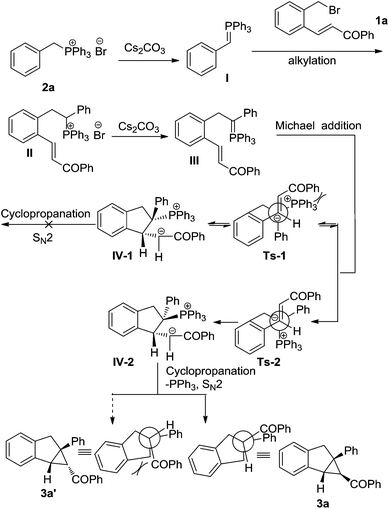
The benzo[3.1.0]carbobicycles are widely distributed as key skeletons in a variety of natural products and biologically active compounds,1,2 as well as versatile building blocks in organic synthesis.3 Considerable efforts have been devoted to forge this core structure.4 Cyclopropanation of cyclic alkenes with metal carbenes is one of the most direct protocols and has been intensively studied.5 However, current limitations of this process include the difficulty associated with the cyclopropanation of trisubstituted olefins, alongside the paucity of stereocontrol of the cyclopropanation, probably because the metal carbene might be very sensitive to the steric hindrance of the alkene.6 An alternative strategy of tandem Michael addition/nucleophilic substitution of ylides, including sulfur, nitrogen and phosphorus, etc., to α,β-unsaturated compounds has been developed to produce cyclopropanes in impressive elegance.7,8 In the construction of trisubstituted benzo[3.1.0]carbobicycles, intramolecular reactions of chalcone derived benzyl bromides in terms of sulfur ylide methods have presented powerful potential (53% yield, Scheme 1, eqn (1)).8l However, in the cases of tetrasubstituted benzo[3.1.0]carbobicycles, relevant studies become difficult because the corresponding secondary benzyl bromides are unstable. Recently, we developed a cascade strategy and found that alkylation of phosphorus ylide provided a facile access to stereo hindered phosphonium salts,9 which could be easily deprotonated to in situ generate the corresponding phosphorus ylide. Followed by typical tandem Michael addition/nucleophilic substitution, a tetrasubstituted benzo[3.1.0]carbobicycle was constructed (Scheme 1, eqn (2)). Herein, we wish to report this reaction in detail.
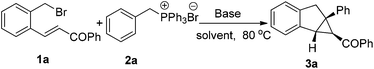
Initial studies focused upon synthesis of benzo[3.1.0]bicycloalkanes via alkylation/cyclopropanation cascade reactions of chalcone-derived benzyl bromide 1a and benzyltriphenylphosphonium bromide 2a (Table 1). Treatment of 2a with Cs2CO3 and 1a at 80 °C in DME (dimethyl ether) led to the alkylation of the in situ generated phosphorus ylide, with subsequent intramolecular cyclization giving 3a in 58% yield with an exclusive cis10 diastereoselectivity (entry 1). Using toluene and ethyl acetate as solvents, the reaction could also proceed in moderate yields (entries 2 and 3). The effect of using a protic solvent was explored, and 55% yield was obtained (entry 4). Both 1,2-dichloroethane and DMF could raise the yield to 70% (entries 5 and 6). When acetonitrile was employed as the solvent, an obvious acceleration of the reaction was observed, and the yield was increased to 82% after 13 hours (entry 7). Alternative inorganic bases such as Na2CO3, K2CO3, and NaOH were also examined, and typically a stronger base led to a higher reactivity (entries 8–10). However, when a much stronger base like t-BuOK was used, the reaction becomes complicated, only 6% of the desired product was obtained (entry 11). In addition, an organic base such as DBU resulted in rather low yield after 20 hours (4% yield, entry 12).
| Entry | Base | Solvent | t (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (0.6 mmol), base (1.8 mmol), in solvent (4 mL). b Isolated yield. | ||||
| 1 | Cs2CO3 | DME | 18 | 58 |
| 2 | Cs2CO3 | Toluene | 14 | 58 |
| 3 | Cs2CO3 | EA | 18 | 53 |
| 4 | Cs2CO3 | t BuOH | 23 | 55 |
| 5 | Cs2CO3 | DCE | 14 | 70 |
| 6 | Cs2CO3 | DMF | 17 | 70 |
| 7 | Cs2CO3 | CH3CN | 13 | 82 |
| 8 | Na2CO3 | CH3CN | 13 | Trace |
| 9 | K2CO3 | CH3CN | 23 | 64 |
| 10 | NaOH | CH3CN | 13 | 78 |
| 11 | t-BuOK | CH3CN | 19 | 6 |
| 12 | DBU | CH3CN | 20 | 4 |
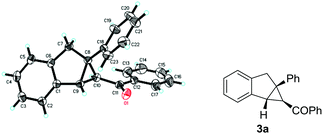
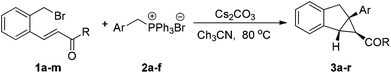
With the optimized reaction conditions in hand, we next investigated the generality of this tandem cyclization reaction, as shown in Table 2. A range of chalcone derivatives 1a–h with different substituents on the benzoyl group, including 4-tBu-, 4-F-, 3-Br, 2-Br, etc., were reacted with 2a smoothly to give the corresponding cyclopropanes 3a–h in good to high yields, and neither electronic changes nor substitution positions on the aryl ring had a significant effect on the reactivities (64–85% yields, entries 1–8). Heterocyclic substrates 1i and 1j, containing 2-furyl and 2-thienyl, both can be converted to the desired products in 70% and 81% yields, respectively (entries 9 and 10). Acetyl and pivaloyl products 3k and 3l could be accomplished facilely in good yields (71% and 83% yields, entries 11 and 12). When cinnamoyl substrate 1m was examined, the indanyl cyclopropane 3m was furnished in moderate yield (entry 13). Remarkably, the current catalytic system is also compatible with a series of phosphonium salts, bearing aryl, heteroaryl and fused-aryl groups. For example, in the case of 4-Br and 3-MeO substituted benzyltriphenylphosphoniums, the reactions showed good to high reactivity (entries 14 and 15). 2-Furyl and 2-thienyl phosphoniums were well tolerated in this tandem cyclization reaction at mild reaction temperatures (entries 16 and 17). With 2-naphthyl phosphonium salt, 3r was afforded in 81% yield after 13 hours (entry 18). Remarkably, in all cases, the diastereoselectivity of these reactions was excellent, only one diastereomer 3a–r was observed. The stereochemistry of 3a was identified by X-ray analysis. As shown in Fig. 1, the torsion angle of C(18)–C(8)–C(10)–C(11) in 3a is 8.2°, and the torsion angle of C(1)–C(9)–C(10)–C(11) in 3a is −124.5°, which indicate that the relative configuration of 3a is cis.11
| Entry | R | Ar | Product | t (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.3 mmol), 2 (0.6 mmol), Cs2CO3 (1.8 mmol), CH3CN (4 mL). b Isolated yield. c 20 °C. d 40 °C. | |||||
| 1 | Ph | Ph | 3a | 14 | 82 |
| 2 | 4-tBuC6H4 | Ph | 3b | 16 | 78 |
| 3 | 4-MeOC6H4 | Ph | 3c | 16 | 85 |
| 4 | 4-FC6H4 | Ph | 3d | 12 | 82 |
| 5 | 4-ClC6H4 | Ph | 3e | 14 | 65 |
| 6 | 4-BrC6H4 | Ph | 3f | 12 | 65 |
| 7 | 3-BrC6H4 | Ph | 3g | 16 | 64 |
| 8 | 2-BrC6H4 | Ph | 3h | 16 | 73 |
| 9 | 2-Furyl | Ph | 3i | 10 | 70 |
| 10 | 2-Thienyl | Ph | 3j | 10 | 81 |
| 11 | Me | Ph | 3k | 12 | 71 |
| 12 | t Bu | Ph | 3l | 12 | 83 |
| 13 | Styryl | Ph | 3m | 13 | 57 |
| 14 | Ph | 4-BrC6H4 | 3n | 18 | 82 |
| 15 | Ph | 3-MeOC6H4 | 3o | 18 | 90 |
| 16c | Ph | 2-Furyl | 3p | 20 | 75 |
| 17d | Ph | 2-Thienyl | 3q | 22 | 52 |
| 18 | Ph | 2-Naphthyl | 3r | 13 | 81 |
A possible mechanism for the tandem cyclization reactions is proposed as shown in Scheme 2. The benzyltriphenylphosphonium bromide 2a was initially treated with Cs2CO3 to in situ generate phosphorus ylide, followed by alkylation with 1a to give the corresponding phosphonium salt. In the presence of Cs2CO3, the phosphonium salt II was deprotonated, followed by an intramolecular conjugate addition of ylide III and a SN2 nucleophilic cyclopropanation, furnishing bicycloalkane 3a and releasing Ph3P. The reaction is stereospecific in all cases that we have studied. It was proposed that the transition-state TS-2 might be more stable than the transition-state TS-1 due to the steric effects between the triphenylphosphine group and the benzoyl group in TS-2. Thus, the formation of an intermediate IV-2 is favored over that of an intermediate IV-1. Furthermore, because of the repulsion between the indanyl group and the benzoyl group, compound 3a was furnished as the major product.
Conclusions
In summary, we have developed a facile approach for the synthesis of benzo[3.1.0]bicycloalkanes via alkylation/cyclopropanation cascade reactions of benzyl bromides with triphenylphosphonium bromide. By elaborate designing of starting materials, this multistep cyclization proceeded smoothly with a wide range of substrate scopes, providing the benzo[3.1.0]bicycloalkanes in 52–90% yields with exclusive diastereoselectivity. Further investigations into this process are currently ongoing in our laboratory.Acknowledgements
We are grateful for the financial support from the National Natural Sciences Foundation of China (Nos. 21121062 and 20932008), the Chinese Academy of Sciences. We thank Dr Xue-Bing Leng (SIOC) and Mr Jie Sun (SIOC) for X-ray crystal analysis. Dedicated to Professor Max Malacria on the occasion of his 65th birthday.Notes and references
- (a) W. L. Lee and M. J. Miller, J. Org. Chem., 2004, 69, 4516 CrossRef CAS PubMed; (b) D. Colombo, E. Modica, G. Bombieri, N. Marchini, R. Lenna and A. Scala, Steroids, 2006, 71, 745 CrossRef CAS PubMed; (c) A. Pauli, Med. Res. Rev., 2006, 26, 223 CrossRef CAS PubMed; (d) L. Acebey, A. Gimenez, L. Acebey, S. Chevalley, Y. Estevez, V. Jullian, C. Moulis, M. Sauvain, A. Valentin, L. Acebey, S. Chevalley, Y. Estevez, V. Jullian, C. Moulis, M. Sauvain, A. Valentin, D. Sereno and S. Beck, Planta Med., 2010, 76, 365 CrossRef CAS PubMed.
- (a) C. S. Vairappan, T. Kawamoto, M. Suzuki, H. Miwa, T. Kawamoto and M. Suzuki, Planta Med., 2004, 70, 1087 CrossRef CAS PubMed; (b) K. P. Madauss, E. L. Stewart and S. P. Williams, Med. Res. Rev., 2007, 27, 374 CrossRef CAS PubMed.
- (a) A. C. Glass, S.-Y. Liu, B. B. Morris and L. N. Zakharov, Org. Lett., 2008, 10, 4855 CrossRef CAS PubMed; (b) H. Tsuchida, M. Tamura and E. Hasegawa, J. Org. Chem., 2009, 74, 2467 CrossRef CAS PubMed.
- For leading references, see: (a) D. Yang, Q. Gao, C.-S. Lee and K.-K. Cheung, Org. Lett., 2002, 4, 3271 CrossRef CAS PubMed; (b) M. Honma, T. Sawada, Y. Fujisawa, M. Utsugi, H. Watanabe, A. Umino, T. Matsumura, T. Hagihara, M. Takano and M. Nakada, J. Am. Chem. Soc., 2003, 125, 2860 CrossRef CAS PubMed; (c) Y. Harrak, C. Blaszykowski, M. Bernard, K. Cariou, E. Mainetti, V. Mouriès, A.-L. Dhimane, L. Fensterbank and M. Malacria, J. Am. Chem. Soc., 2004, 126, 8656 CrossRef CAS PubMed; (d) M. R. Luzung, J. P. Markham and F. D. Toste, J. Am. Chem. Soc., 2004, 126, 10858 CrossRef CAS PubMed; (e) T. Miura, T. Sasaki, T. Harumashi and M. Murakami, J. Am. Chem. Soc., 2006, 128, 2516 CrossRef CAS PubMed; (f) J. A. Bull and A. B. Charette, J. Am. Chem. Soc., 2010, 132, 1895 CrossRef CAS PubMed; (g) R. R. Nani and S. E. Reisman, J. Am. Chem. Soc., 2013, 135, 7304 CrossRef CAS PubMed.
- For reviews, see: (a) M. P. Doyle and D. C. Forbes, Chem. Rev., 1998, 98, 911 CrossRef CAS PubMed; (b) H. Lebel, J. F. Marcoux, C. Molinaro and A. B. Charette, Chem. Rev., 2003, 103, 977 CrossRef CAS PubMed; (c) H. Pellissier, Tetrahedron, 2008, 64, 7041 CrossRef CAS PubMed; (d) Z. Zhang and J. Wang, Tetrahedron, 2008, 64, 6577 CrossRef CAS PubMed.
- (a) H. Suematsu, S. Kanchiku, T. Uchida and T. Katsuki, J. Am. Chem. Soc., 2008, 130, 10327 CrossRef CAS PubMed; (b) C. Deng, L.-J. Wang, J. Zhu and Y. Tang, Angew. Chem., Int. Ed., 2012, 51, 11620 CrossRef CAS PubMed; (c) J. Li, S.-H. Liao, H. Xiong, Y.-Y. Zhou, X.-L. Sun, Y. Zhang, X.-G. Zhou and Y. Tang, Angew. Chem., Int. Ed., 2012, 51, 8838 CrossRef CAS PubMed; (d) Z.-Y. Cao, X. Wang, C. Tan, X.-L. Zhao, J. Zhou and K. Ding, J. Am. Chem. Soc., 2013, 135, 8197 CrossRef CAS PubMed.
- For reviews of ylide involved cyclopropanation, see: (a) A. H. Li, L. X. Dai and V. K. Aggarwal, Chem. Rev., 1997, 97, 2341 CrossRef CAS PubMed; (b) L. X. Dai, X. L. Hou and Y. G. Zhou, Pure Appl. Chem., 1999, 71, 369 CAS; (c) see: ref. 5b; ; (d) Y. Tang, S. Ye and X. L. Sun, Synlett, 2005, 2720 CrossRef CAS PubMed; For recent examples of cyclopropanation with ylide reagents, see: (e) R. K. Kunz and D. W. C. MacMillan, J. Am. Chem. Soc., 2005, 127, 3240 CrossRef CAS PubMed; (f) Y. H. Zhao, G. Zhao and W. G. Cao, Tetrahedron: Asymmetry, 2007, 18, 2462 CrossRef CAS PubMed; (g) C. Y. Zhu, X. Y. Cao, B. H. Zhu, C. Deng, X. L. Sun, B. Q. Wang, Q. Shen and Y. Tang, Chem. – Eur. J., 2009, 15, 11465 CrossRef CAS PubMed; (h) R. Appel, N. Hartmann and H. Mayr, J. Am. Chem. Soc., 2010, 132, 17894 CrossRef CAS PubMed; (i) S. L. Riches, C. Saha, N. F. Filgueira, E. Grange, E. M. McGarrigle and V. K. Aggarwal, J. Am. Chem. Soc., 2010, 132, 7626 CrossRef CAS PubMed; (j) B. H. Zhu, R. Zhou, J. C. Zheng, X. M. Deng, X. L. Sun, Q. Shen and Y. Tang, J. Org. Chem., 2010, 75, 3454 CrossRef CAS PubMed; (k) Y. Cheng, J. An, L. Q. Lu, L. Luo, Z. Y. Wang, J. R. Chen and W. J. Xiao, J. Org. Chem., 2011, 76, 281 CrossRef CAS PubMed; (l) R. Zhou, X. M. Deng, J. C. Zheng, Q. Shen, X. L. Sun and Y. Tang, Chin. J. Chem., 2011, 29, 995 CrossRef CAS PubMed; (m) A. Biswas, S. De Sarkar, L. Tebben and A. Studer, Chem. Commun., 2012, 48, 5190 RSC; (n) L. Q. Lu, Z. H. Ming, J. An, C. Li, J. R. Chen and W. J. Xiao, J. Org. Chem., 2012, 77, 1072 CrossRef CAS PubMed; (o) C. Q. Wang, Tetrahedron Lett., 2012, 53, 7003 CrossRef CAS PubMed; For examples of cyclopropanation using stoichiometric phosphorus ylides, see: (p) J. Wang, X. H. Liu, S. X. Dong, L. L. Lin and X. M. Feng, J. Org. Chem., 2013, 78, 6322 CrossRef CAS PubMed; (q) A. Krief, L. Provins and A. Froidbise, Tetrahedron Lett., 1998, 39, 1437 CrossRef CAS; (r) O. I. Kolodiazhnyi, Phosphorus Ylides: Chemistry and Application in Organic Synthesis, Wiley-VCH, New York, 1999 Search PubMed; (s) S. Ahmad, L. M. Doweyko, S. Dugar, N. Grazier, K. Ngu, S. C. Wu, K. J. Yost, B. C. Chen, J. Z. Gougoutas, J. D. DiMarco, S. J. Lan, B. J. Gavin, A. Y. Chen, C. R. Dorso, R. Serafino, M. Kirby and K. S. Atwal, J. Med. Chem., 2001, 44, 3302 CrossRef CAS PubMed; (t) E. Bunuel, S. D. Bull, S. G. Davies, A. C. Garner, E. D. Savory, A. D. Smith, R. J. Vickers and D. J. Watkin, Org. Biomol. Chem., 2003, 1, 2531 CAS; (u) S. Redon, S. Leleu, X. Pannecoucke, X. Franck and F. Outurquin, Tetrahedron, 2008, 64, 9293 CrossRef CAS PubMed.
- For reviews on catalytic ylide cyclopropanation, see: (a) S.-H. Liao, P. Wang and Y. Tang, Covalent Activations: Ylides in Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications, Wiley-VCH & Sons, Weinheim, Germany, 2013 Search PubMed; (b) E. M. McGarrigle, E. L. Myers, O. Illa, M. A. Shaw, S. L. Riches and V. K. Aggarwal, Chem. Rev., 2007, 107, 5841 CrossRef CAS PubMed; (c) I. S. del Villar, A. Gradillas, G. Dominguez and J. Perez-Castells, Tetrahedron Lett., 2010, 51, 3095 CrossRef CAS PubMed; For examples of ylide catalyzed cyclopropanation, see: (d) Y. Z. Huang, Y. Tang, Z. L. Zhou, W. Xia and L. P. Shi, J. Chem. Soc., Perkin Trans. 1, 1994, 893 RSC; (e) V. K. Aggarwal, H. W. Smith, G. Hynd, R. V. H. Jones, R. Fieldhouse and S. E. Spey, J. Chem. Soc., Perkin Trans. 1, 2000, 3267 RSC; (f) C. D. Papageorgiou, S. V. Ley and M. J. Gaunt, Angew. Chem., Int. Ed., 2003, 42, 828 CrossRef CAS PubMed; (g) W. W. Liao, K. Li and Y. Tang, J. Am. Chem. Soc., 2003, 125, 13030 CrossRef CAS PubMed; (h) N. Bremeyer, S. C. Smith, S. V. Ley and M. J. Gaunt, Angew. Chem., Int. Ed., 2004, 43, 2681 CrossRef CAS PubMed; (i) C. D. Papageorgiou, M. A. C. de Dios, S. V. Ley and M. J. Gaunt, Angew. Chem., Int. Ed., 2004, 43, 4641 CrossRef CAS PubMed; (j) X. M. Deng, P. Cai, S. Ye, X. L. Sun, W. W. Liao, K. Li, Y. Tang, Y. D. Wu and L. X. Dai, J. Am. Chem. Soc., 2006, 128, 9730 CrossRef CAS PubMed; (k) C. C. C. Johansson, N. Bremeyer, S. V. Ley, D. R. Owen, S. C. Smith and M. J. Gaunt, Angew. Chem., Int. Ed., 2006, 45, 6024 CrossRef CAS PubMed; (l) L. W. Ye, X. L. Sun, C. Y. Li and Y. Tang, J. Org. Chem., 2007, 72, 1335 CrossRef CAS PubMed; (m) H. Jiang, X. L. Sun, C. Y. Zhu, L. X. Dai and Y. Tang, Tetrahedron, 2008, 64, 5032 CrossRef CAS PubMed.
- For examples of alkylation of phosphorus ylide, see: (a) H. J. Bestmann and H. Schulz, Chem. Ber., 1962, 95, 2921 CrossRef CAS PubMed; (b) S. Tripett, J. Chem. Soc., 1962, 4733 Search PubMed; (c) H. J. Bestmann, H. Haberlein and W. Eisele, Chem. Ber., 1966, 99, 28 CrossRef CAS PubMed; (d) E. Werner and E. Zbiral, Angew. Chem., 1967, 79, 899 CrossRef PubMed; (e) H. W. Heine, H. B. Lowrit and K. C. Irving, J. Org. Chem., 1970, 35, 444 CrossRef CAS; (f) C. J. Dewlin and B. J. Walker, Tetrahedron, 1972, 28, 3501 CrossRef; (g) H. Schmidbaur, G. Blaschke, B. Zimmergasser and U. Schubert, Chem. Ber., 1980, 113, 1612 CrossRef PubMed; (h) A. Schier and H. Schmidbaur, Chem. Ber., 1984, 117, 2314 CrossRef CAS PubMed; (i) H. Schmidbaur, A. Schier, C. M. F. Frazao and G. Muller, J. Am. Chem. Soc., 1986, 108, 976 CrossRef CAS; (j) A. R. Katrizki, J. Jiang and J. Greenhill, J. Org. Chem., 1993, 58, 19879 Search PubMed; (k) P. Wang, S. Liao, J.-B. Zhu and Y. Tang, Chem. Commun., 2014, 50, 808 RSC.
- The cis isomer is defined by the phenyl group and the benzoyl group substituted on cyclopropane, which are on the same side of the cyclopropane ring.
- CCDC 962017 (3a) contains the supplementary crystallographic data for this paper.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and the characterization data of new compounds. CCDC 962017. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qo00134f |
| This journal is © the Partner Organisations 2014 |