Sterically demanding aryl–alkyl Suzuki–Miyaura coupling†
Abstract
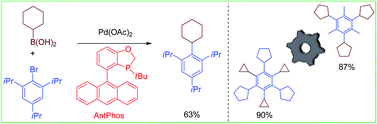
Efficient sterically demanding aryl–alkyl Suzuki–Miyaura coupling between di-ortho-substituted aryl halides and secondary alkylboronic acids has been achieved with a Pd-AntPhos catalyst that has shown high reactivity and a broad substrate scope with unprecedented steric hindrance. The methodology has facilitated the synthesis of molecular gears such as by cross-coupling.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers for 2014

 Please wait while we load your content...
Please wait while we load your content...