Copper-catalyzed three-component synthesis of 5-acetamidoimidazoles from carbodiimides, acyl chlorides and isocyanides†
Giulia
Sapuppo
a,
Qian
Wang
a,
Dominique
Swinnen‡
b and
Jieping
Zhu
*a
aLaboratory of Synthesis and Natural Products, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304, CH-1015 Lausanne, Switzerland. E-mail: jieping.zhu@epfl.ch; Fax: (+41) 21-693-97-40
bMerck Serono, S. A., 9 Chemin des Mines, 1211 Geneva 20, Switzerland
First published on 28th February 2014
Abstract
Only very limited methods are available for the synthesis of 5-amino imidazoles. We report in this paper that three-component reaction of acyl chlorides, carbodiimides and α-isocyanoacetates or TosMIC took place smoothly in the presence of Et3N (2.5 equiv.) and a catalytic amount of copper iodide (0.2 equiv.) to afford 1,4-disubstituted 5-acetamidoimidazoles in good yields.
Introduction
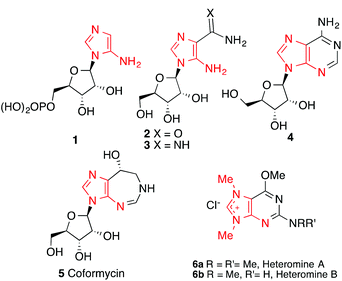
Functionalized imidazole-containing compounds have found a broad range of applications in pharmaceuticals and in organic synthesis.1,2 Among them, the 5-aminoimidazole, a sub-class of this important family of heterocycles, is present in some biologically important natural products and medicinally relevant synthetic compounds. For example, 5-aminoimidazole ribonucleotide (AIR) (1) is an essential intermediate in the de novo biosynthesis of purine ribonucleotides3 and thiamin.4 5-Amino-4-carboxamide-1-β-D-ribofuranoside (AICAR) (2) is a commonly used AMP-kinase activator employed to treat and protect against cardiac ischemic injury.5 The arabinosyl amidine 3 is a synthetic precursor and potential prodrug of 9-β-D-arabinofuranosyladenine (4, Ara-A), a valuable anti-tumor drug.6 The amino-imidazole motif is also found in important natural products such as coformycin (5), a potent inhibitor of adenosine deaminase with anticancer and antiviral properties (Fig. 1).7 Obviously, substituted 5-aminoimidazoles are also useful intermediates in the synthesis of purine,8 and the purine-derived natural products. For example, heteromine A (6a) and B (6b),9 cytotoxic against several cancer cell lines, have been synthesized from 5-amino-4-cyanoimidazole.10 Additionally, it is interesting to note that condensation of 5-amino-4-cyanoimidazole with formamidine has been proposed as a possible prebiotic pathway to adenine.11In light of the importance of the functionalized imidazoles, many synthetic methods have been developed for the construction of this heterocycle as well as for the regio-selective functionalization of the imidazole ring.1 However, only very limited methods are currently available for the synthesis of 5-aminoimidazoles. Among them, the three-step synthesis from α-aminomalonitriles, orthoformates and primary amines developed by Shaw and co-workers has been widely adopted by synthetic and medicinal chemists.6,8,12 Alternatively, Bartlett developed an elegant synthesis of 5-amino-imidazole-4-carboxylic ester by condensation of isothioureas with the pre-formed enolate of ethyl isocyanoacetate (five examples with yields ranging from 30 to 75%).13
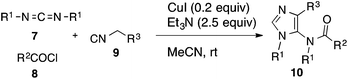
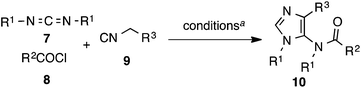
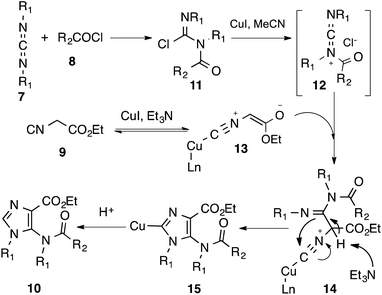
The lack of synthetic methodologies to 5-amino-imidazole stimulated our interest in developing a one-pot synthesis of this important heterocycle. We report herein a CuI-catalyzed three-component reaction of carbodiimides 7, acyl chlorides 8 and isocyanides 9 for the synthesis of 1,4-disubstituted 5-acetamidoimidazoles 10 in good yields (Scheme 1).14,15
Results and discussion
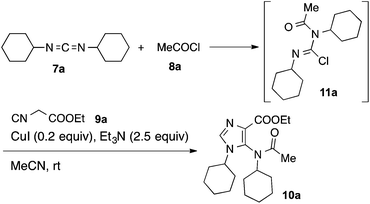
By virtue of the carbene-like reactivity of its divalent carbon, isocyanides are key components used in the development of classic Passerini-3CR,16 Ugi-4CR17 and their variants thereof.18 On the other hand, carbodiimides19 have only scarcely been exploited in multicomponent reactions, although they are also capable of reacting with both nucleophiles and electrophiles.20 In connection with our ongoing project on the development of isocyanide-based multicomponent reactions,21 we became interested in investigating the reaction of isocyanides, carbodiimides in the presence of external electrophiles and as a prelude, acyl chlorides were chosen for the present study.N,N′-Dicyclohexylcarbodiimide (DCC, 7a), acetyl chloride (8a) and ethyl α-isocyanoacetate (9a) were selected as test substrates to survey the reaction conditions. It is known that carbodiimides can be pre-activated by acyl chlorides,22 facilitating the subsequent nucleophilic addition. Recent work from Zhang and Xi has provided convincing evidence that the reaction between 7 and 8 afforded isolable N-acyl chloroformamidine 11 that is susceptible to react with a range of nucleophiles including water, 1,3-dicarbonyls, terminal alkynyl, (thio)urea, sodium hydrosulfide etc.23 Since isocyanide can also react with acyl chloride spontaneously,24 the addition order of substrates is therefore an important factor that will determine the outcome of the reaction. In the present study, a protocol involving first mixing of 7a and 8a followed by addition of 9a was adopted. While 7a and 8a reacted spontaneously to produce 11a, conditions for its subsequent reaction with 9a needed to be carefully optimized. Reaction of the isolated N-acyl chloroformamidine 11a with the pre-formed enolate of 9a afforded the desired heterocycle in only low to moderate yield (≤40%) regardless of the nature of the base (Et3N, DBU, K2CO3, Li2CO3, Cs2CO3, CsF, NaH, BuLi, LHMDS, KHMDS) and the solvent (DCM, MeCN, THF, DMSO) used. We then turned our attention to the Lewis acid-catalyzed (mediated) conditions.25,26 After having screened the metal salts (AgOTf, Ag3PO4, CuO, CuSO4, Cu(OAc)2, CuCl, CuBr, CuI), the solvents (DCM, MeCN, THF, DMSO), the additives (TMEDA, DABCO, HMPA, 18-C-6) and the temperature, the optimum conditions found consisted of mixing 7a and 8a at 0 °C for 20 min followed by addition of ethyl isocyanoacetate (9a) in MeCN (c = 0.3 M), CuI (0.2 equiv.) and Et3N (2.5 equiv.) at room temperature. Under these conditions, we were able to isolate 10a in 77% yield (Scheme 2).
The scope of the reaction was next investigated (Table 1). Both DCC and DIC can be successfully used for this reaction. Primary and secondary aliphatic acid chlorides incorporating a variety of functional groups (alkyl halides, conjugated or isolated double bond, ester) were well-accepted substrates furnishing the imidazoles in good yields. However, the aromatic acid chlorides provided the imidazoles with reduced yields (entries 13, 14). t-Butyl α-isocyanoacetate participated also in this reaction (entry 18) giving 10r in moderate yield. p-Toluenesulfonylmethyl isocyanide (TosMIC) participated in this 3CR as well to afford the corresponding 1-alkyl-4-tosyl-5-acetamidoimidazoles (entries 19, 20).
| Entry | R1 | R2 | R3 | Product | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: carbodiimide (0.5 mmol), RCOCl (1.05 equiv.), 0 °C and then isocyanide (2.0 equiv.), CuI (0.2 equiv.), Et3N (2.5 equiv.), MeCN (c 0.3 M), room temperature, 6 h. b Isolated yield after FCC on silica gel. | |||||
| 1 | Cy | Me | CO2Et | 10a | 77 |
| 2 | iPr | Me | CO2Et | 10b | 75 |
| 3 | iPr | Et | CO2Et | 10c | 67 |
| 4 | Cy | nC5H11 | CO2Et | 10d | 67 |
| 5 | iPr | CH2CMe3 | CO2Et | 10e | 71 |
| 6 | Cy | (CH2)2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2 |
CO2Et | 10f | 65 |
| 7 | Cy | iPr | CO2Et | 10g | 66 |
| 8 | iPr | Cy | CO2Et | 10h | 73 |
| 9 | iPr | Cyclopentyl | CO2Et | 10i | 61 |
| 10 | Cy | Bn | CO2Et | 10j | 41 |
| 11 | Cy | CH2OPh | CO2Et | 10k | 22 |
| 12 | iPr | CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHMe CHMe |
CO2Et | 10l | 69 |
| 13 | Cy | Ph | CO2Et | 10m | 47 |
| 14 | iPr | 4-BrC6H4 | CO2Et | 10n | 25 |
| 15 | Cy | (CH2)2CH2Cl | CO2Et | 10o | 68 |
| 16 | iPr | (CH2)4CH2Br | CO2Et | 10p | 80 |
| 17 | iPr | (CH2)2CO2Me | CO2Et | 10q | 42 |
| 18 | Cy | Me | CO2tBu | 10r | 39 |
| 19 | Cy | Me | Ts | 10s | 82 |
| 20 | Cy | (CH2)2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2 |
Ts | 10t | 68 |
Upon mixing an equal molar of 11a with CuI, we observed a rapid disappearance of 11a leading to a polar compound that we assumed to be the N-acylimminium salt 12.27 Addition of ethyl α-isocyanoacetate 9a to the reaction mixture led to the formation of 10a. Based on this control experiment, we proposed the following reaction pathway to account for the formation of 1,4-disubstituted 5-acetamidoimidazoles 10 (Scheme 3). Reaction of carbodiimide 7 with acyl chloride 8 gave the N-acyl chloroformamidine 11 that was converted to a hypothetic N-acylimminium salt 12 upon addition of CuI. On the other hand, deprotonation of the copper(I)-coordinated ethyl α-isocyanoacetate yielded enolate 13. Nucleophilic addition of 13 to 12 afforded an intermediate 14.28 Deprotonation of the remaining α-protons followed by intramolecular attack of the resulting secondary amine to the isonitrile carbon provided, after protonation, the observed imidazole 10.
Conclusion
In summary, we developed a novel CuI-catalyzed three-component reaction of isocyanides, carbodiimides and acyl chlorides for the synthesis of 5-acetamidoimidazoles. To the best of our knowledge, this represented the first examples of one-pot synthesis of this type of imidazole. We are further exploiting the reactivity of these unique heterocycles for accessing other more complex heterocyclic scaffolds.29Experimental
Mass spectra were determined with a Waters ACQUITY H-class UPLC/MS ACQ-SQD by electron ionisation (EI positive and negative) or with a Finnigan TSQ7000 by electrospray ionization (ESI+). The accurate masses were calculated by the mass spectrometry service of the EPFL by ESI-TOF using a QTOF Ultima from Waters. NMR spectra were recorded on a Brüker Avance III-400, Brüker Avance-400 or Brüker DPX-400 spectrometer at room temperature, the 1H frequency is at 400.13 MHz, and the 13C frequency is at 100.62 MHz. Chemical shifts δ) are reported in parts per million (ppm) from tetramethylsilane. NMR experiments were carried out in deuterochloroform (CDCl3). The following abbreviations are used for the multiplicities: s: singlet, d: doublet, m: multiplet for proton spectra. Coupling constants (J) are reported in hertz (Hz). IR spectra were recorded on a Jasco FT/IR-4100 spectrometer outfitted with the PIKE technology. Melting points were determined using a Stuart SMP30 and were uncorrected. Flash column chromatography was performed using Silicycle silica gel: 230–400 mesh (40–63 μm). Reactions were monitored using Merck Kieselgel 60 F254 on aluminium sheets. TLCs were visualized by UV fluorescence (254 nm) and then with one of the following: KMnO4, ninhydrin, pancaldi, p-anisaldehyde or vanillin. All reagents were obtained from commercial suppliers unless otherwise stated.General procedure for the three-component synthesis of 1,4-disubstituted 5-acetamidoimidazoles
Acyl chloride 8 (1.05 equiv.) was added dropwise to carbodiimide 7 (0.5 mmol) under Ar and the mixture was stirred at 0 °C for 20 min. To the reaction mixture were added a solution of α-isocyanoacetate 9 (2.0 equiv.) in acetonitrile (c, 0.3 M), CuI (0.2 equiv.) and Et3N (2.5 equiv.), successively. After being stirred at room temperature for 2–6 h, the reaction was quenched by addition of aqueous NH4OH (2.0 M) and the aqueous phase was extracted with EtOAc. The combined organic phases were washed with brine, dried over Na2SO4 and concentrated under vacuum. The residue was purified by flash chromatography on silica gel (DCM–EtOAc) to afford 5-acetamidoimidazole 10.Acknowledgements
We thank Merck Serono, EPFL (Switzerland) for financial support.Notes and references
- (a) N. X. Huang and L. Liu, in Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzy, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Pergamon, Oxford, 2008, vol. 4, pp. 143–364 Search PubMed; (b) F. Bellina, S. Cauteruccio and R. Rossi, Tetrahedron, 2007, 63, 4571–4624 CrossRef CAS PubMed; (c) S. Kaniyo and Y. Yamamoto, Chem.–Asian J., 2007, 2, 568–578 CrossRef PubMed; (d) F. Bellina and R. Rossi, Adv. Synth. Catal., 2010, 352, 1223–1276 CrossRef CAS.
- Imidazolium-based ionic liquids: J. Dupont, R. F. de Souza and P. A. Suarez, Chem. Rev., 2002, 102, 3667–3692 CrossRef CAS PubMed.
- (a) J. L. Schrimsher, F. J. Schendel and J. Stubbe, Biochemistry, 1986, 25, 4356–4365 CrossRef CAS; (b) J. L. Schrimsher, F. J. Schendel, J. Stubbe and J. M. Smith, Biochemistry, 1986, 25, 4366–4371 CrossRef CAS.
- B. Estramareix and S. David, Biochem. Biophys. Res. Commun., 1986, 134, 1136–1141 CrossRef CAS.
- H. R. Samari and P. O. Seglen, J. Biol. Chem., 1998, 273, 23758–23763 CrossRef CAS PubMed.
- K. Kadir, G. Shaw and D. Wright, J. Chem. Soc., Perkin Trans. 1, 1980, 2728–2731 RSC.
- A. Reayi and R. S. Hosmane, J. Med. Chem., 2004, 47, 1044–1050 CrossRef CAS PubMed.
- M. J. Alves, B. L. Booth, M. Fernanda and J. R. P. Proenç, J. Chem. Soc., Perkin Trans. 1, 1990, 1705–1712 RSC.
- (a) Y. L. Lin, H. P. Lee, J. C. On and Y. H. Kuo, Heterocycles, 1996, 43, 781–786 CrossRef CAS PubMed; (b) Y.-L. Lin, R.-L. Huang, C.-M. Chang and Y.-H. Kuo, J. Nat. Prod., 1997, 60, 982–985 CrossRef CAS PubMed.
- (a) C. Peinador, J. M. Quintela and M. J. Moreira, Tetrahedron, 1997, 53, 8269–8272 CrossRef CAS; (b) H. Roggen, C. Charnock and L.-L. Gundersen, Tetrahedron, 2009, 65, 5199–5203 CrossRef CAS PubMed.
- (a) J. P. Ferris and L. E. Orgel, J. Am. Chem. Soc., 1966, 88, 1074–1074 CrossRef CAS; (b) T. H. Koch and R. M. Rodehorst, J. Am. Chem. Soc., 1974, 96, 6707–6710 CrossRef CAS.
- (a) G. Shaw, R. N. Wanener, R. K. Ralph and D. N. Butler, J. Chem. Soc., 1959, 1648–1655 RSC; (b) G. Mackenzie, H. A. Wilson, G. Shaw and D. Ewing, J. Chem. Soc., Perkin Trans. 1, 1988, 2541–2546 RSC; (c) G. Cristalli, A. Eleuteri, P. Franchetti, M. Grifantini, S. Vittori and G. Lupidi, J. Med. Chem., 1991, 34, 1187–1192 CrossRef CAS; (d) M. J. Alves, L. B. Booth, A. P. Freitas and M. F. J. R. P. Proença, J. Chem. Soc., Perkin Trans. 1, 1992, 913–917 RSC; (e) B. L. Booth, A. M. Dias and M. F. Proença, J. Chem. Soc., Perkin Trans. 1, 1992, 2119–2126 RSC; (f) P. R. Birkett, H. King, C. B. Chapleo, D. F. Ewing and G. MacKenzie, Tetrahedron, 1993, 49, 11029–11036 CrossRef CAS; (g) F. Corelli, V. Summa, A. Brogi, E. Monteagudo and M. Botta, J. Org. Chem., 1995, 60, 2008–2015 CrossRef CAS; (h) M. McLaughlin, R. M. Mohareb and H. Rapoport, J. Org. Chem., 2003, 68, 50–54 CrossRef CAS PubMed.
- J. T. Hunt and P. Bartlett, Synthesis, 1978, 741–742 CrossRef CAS.
- Copper-catalyzed condensation of α-isocyanoacetate with aromatic isocyanide leading to imidazole has been reported, see: C. Kanazawa, S. Kamijo and Y. Yamamoto, J. Am. Chem. Soc., 2006, 128, 10662–10663 CrossRef CAS PubMed.
- Van Leusen's three-component synthesis of imidazole using TosMIC as a key substrate: (a) A. M. Van Leusen, J. Wildeman and O. H. Oldenziel, J. Org. Chem., 1977, 42, 1153–1156 CrossRef CAS; (b) J. Sisko, A. J. Kassick, M. Mellinger, J. J. Filan, A. Allen and M. A. Olsen, J. Org. Chem., 2000, 65, 1516–1524 CrossRef CAS PubMed; (c) V. Gracias, A. F. Gasiecki and S. W. Djuric, Org. Lett., 2005, 7, 3183–3186 CrossRef CAS PubMed; (d) F. de Moliner and C. Hulme, Org. Lett., 2012, 14, 1354–1357 CrossRef CAS PubMed.
- L. Banfi and R. Riva, in Org. React., ed. A. B. Charette, John Wiley & Sons Inc., 2005, vol. 65, pp. 1–140 Search PubMed.
- A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef.
- For selected reviews on isocyanide-based multicomponent reactions, see: (a) J. Zhu, Eur. J. Org. Chem., 2003, 1133–1144 CrossRef CAS; (b) A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed; (c) L. El Kaim and L. Grimaud, Tetrahedron, 2009, 65, 2153–2171 CrossRef CAS PubMed; (d) L. Banfi, R. Riva and A. Basso, Synlett, 2010, 23–41 CrossRef CAS PubMed; (e) A. V. Gulevich, A. G. Zhdanko, R. V. Orru and V. G. Nenajdenko, Chem. Rev., 2010, 110, 5235–5331 CrossRef CAS PubMed; (f) G. C. Tron, Eur. J. Org. Chem., 2013, 1849–1859 CrossRef CAS.
- For selected reviews on carbodiimides chemistry, see: (a) H. G. Khorana, Chem. Rev., 1953, 53, 145–166 CrossRef CAS; (b) A. Williams and I. T. Ibrahim, Chem. Rev., 1981, 81, 589–636 CrossRef CAS; (c) W.-X. Zhang and Z. Hou, Org. Biomol. Chem., 2008, 6, 1720–1730 RSC.
- Multicomponent reactions involving carbodiimides: (a) X. Xu, D. Cheng, J. Li, H. Guo and J. Yan, Org. Lett., 2007, 9, 1585–1587 CrossRef CAS PubMed; (b) X. Xu, J. Gao, D. Cheng, J. Li, G. Qiang and H. Guo, Adv. Synth. Catal., 2008, 250, 61–64 CrossRef; (c) Z. Wang, Y. Wang, W.-X. Zhang, Z. Hou and Z. Xi, J. Am. Chem. Soc., 2009, 131, 15108–15109 CrossRef CAS PubMed; (d) Y. Wang, W.-X. Zhang, Z. Wang and Z. Xi, Angew. Chem., Int. Ed., 2011, 50, 8122–8126 CrossRef CAS PubMed; (e) H. Wang, S. Ye, H. Jin, J. Liu and J. Wu, Tetrahedron, 2011, 67, 5871–5877 CrossRef CAS PubMed; (f) F. Zhao, Y. Wang, W.-X. Zhang and Z. Xi, Org. Biomol. Chem., 2012, 10, 6266–6270 RSC; (g) G. Qiu, Y. Lu and J. Wu, Org. Biomol. Chem., 2013, 11, 798–802 RSC.
- Selected examples: (a) A. Fayol and J. Zhu, Org. Lett., 2004, 6, 115–118 CrossRef CAS PubMed; (b) A. Fayol and J. Zhu, Org. Lett., 2005, 7, 239–242 CrossRef CAS PubMed; (c) T. Ngouansavanh and J. Zhu, Angew. Chem., Int. Ed., 2006, 45, 3495–3497 CrossRef CAS PubMed; (d) S.-X. Wang, M.-X. Wang, D.-X. Wang and J. Zhu, Org. Lett., 2007, 9, 3615–3618 CrossRef CAS PubMed; (e) S.-X. Wang, M.-X. Wang, D.-X. Wang and J. Zhu, Angew. Chem., Int. Ed., 2008, 47, 388–391 CrossRef CAS PubMed; (f) T. Yue, M.-X. Wang, D.-X. Wang and J. Zhu, Angew. Chem., Int. Ed., 2008, 47, 9454–9457 CrossRef CAS PubMed; (g) J. M. Grassot, G. Masson and J. Zhu, Angew. Chem., Int. Ed., 2008, 47, 947–950 CrossRef CAS PubMed; (h) T. Yue, M.-X. Wang, D.-X. Wang, G. Masson and J. Zhu, J. Org. Chem., 2009, 74, 8396–8399 CrossRef CAS PubMed; (i) W. Erb, N. Neuville and J. Zhu, J. Org. Chem., 2009, 74, 3109–3115 CrossRef CAS PubMed; (j) T. Yue, M.-X. Wang, D.-X. Wang, G. Masson and J. Zhu, Angew. Chem., Int. Ed., 2009, 48, 6717–6721 CrossRef CAS PubMed; (k) C. Lalli, M. J. Bouma, D. Bonne, G. Masson and J. Zhu, Chem.–Eur. J., 2011, 17, 880–889 CrossRef CAS PubMed; (l) Y. Odabachian, S. Tong, Q. Wang, M.-X. Wang and J. Zhu, Angew. Chem., Int. Ed., 2013, 52, 10878–10882 CrossRef CAS PubMed.
- (a) K. Hartke and E. Palou, Chem. Ber., 1966, 99, 3155–3162 CrossRef CAS; (b) K. Hartke, Chem. Ber., 1966, 99, 3163–3172 CrossRef CAS; (c) W. T. Brady and R. A. Owens, J. Org. Chem., 1977, 42, 3220–3222 CrossRef CAS.
- Y. Wang, C. Yue, F. Zhano, W.-X. Zhang and Z. Xi, Synthesis, 2013, 347–354 Search PubMed.
- (a) J. U. Nef, Justus Liebigs Ann. Chem., 1892, 270, 267–335 CrossRef; (b) I. Ugi and U. Fetzer, Chem. Ber., 1961, 94, 1116–1121 CrossRef CAS; (c) M. Westling and T. Livinghouse, J. Am. Chem. Soc., 1987, 109, 590–592 CrossRef CAS; (d) L. El Kaim, L. Grimaud and S. Wagschal, Synlett, 2009, 1315–1317 CrossRef CAS PubMed; (e) A. dos Santos, L. El Kaim, L. Grimaud and C. Ronsseray, Chem. Commun., 2009, 3907–3909 RSC; (f) R. Mossetti, T. Pirali, G. C. Tron and J. Zhu, Org. Lett., 2010, 12, 820–823 CrossRef CAS PubMed; (g) R. Mossetti, D. Caprioglio, G. Colombano, G. C. Tron and T. T. Pirali, Org. Biomol. Chem., 2011, 9, 1627–1631 RSC For a review, see: (h) T. Livinghouse, Tetrahedron, 1999, 48, 2209–2222 Search PubMed.
- For a review, see: (a) A. V. Lygin and A. de Meijere, Angew. Chem., Int. Ed., 2010, 49, 9094–9124 CrossRef CAS PubMed; (b) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257–5269 RSC.
- (a) T. Saegusa, Y. Ito, H. Kinoshita and S. Tomita, J. Org. Chem., 1971, 36, 3316–3323 CrossRef CAS; (b) Y. Ito, M. Sawamura and T. Hayashi, J. Am. Chem. Soc., 1986, 108, 6405–6406 CrossRef CAS; (c) Y.-R. Lin, X.-T. Zhou, L.-X. Dai and J. Sun, J. Org. Chem., 1997, 62, 1799–1803 CrossRef CAS; (d) R. Grigg, M. I. Lansdell and M. Thornton-Pett, Tetrahedron, 1999, 55, 2025–2044 CrossRef CAS; (e) H. Takaya, S. Kojima and S. Murahashi, Org. Lett., 2001, 3, 421–424 CrossRef CAS; (f) S. Kamijo, C. Kanazawa and Y. Yamamoto, J. Am. Chem. Soc., 2005, 127, 9260–9266 CrossRef CAS PubMed; (g) N. Elders, E. Ruijter, F. J. J. de Kanter, M. B. Groen and R. V. A. Orru, Chem.–Eur. J., 2008, 14, 4961–4973 CrossRef CAS PubMed; (h) Y. Odabachian, Q. Wang and J. Zhu, Chem.–Eur. J., 2013, 19, 12229–12233 CrossRef CAS.
- It is difficult to record NMR spectra due to the presence of copper salt in the mixture. Therefore, no spectroscopic data were collected to support the involvement of 12 as an intermediate on the way to 5-acetamidoimidazoles 10.
- The reaction of α-metalated isocyanide with imidoyl chloride has been reported for the synthesis of 4-tosylimidazole: A. M. van Leusen and O. H. Oldenslel, Tetrahedron Lett., 1972, 13, 2373–2374 CrossRef.
- Exploitation of the enamide and the aza-diene properties of 5-aminooxazoles for the synthesis of macrocycles and heterocycles: (a) G. Zhao, X. Sun, H. Bienaymé and J. Zhu, J. Am. Chem. Soc., 2001, 123, 6700–6701 CrossRef CAS; (b) P. Janvier, X. Sun, H. Bienaymé and J. Zhu, J. Am. Chem. Soc., 2002, 124, 2560–2567 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra. See DOI: 10.1039/c4qo00034j |
| ‡ Current address: UCB S.A., Chemin du Foriest, 1420 Braine l'Alleud, Belgium. |
| This journal is © the Partner Organisations 2014 |