Enyne [4 + 4] photocycloaddition with polycyclic aromatics†‡
Buddha B.
Khatri
,
Svitlana
Kulyk§
and
Scott McN.
Sieburth
*
Department of Chemistry, Temple University, 1901 N. 13th Street, Philadelphia, Pennsylvania, 19122 USA. E-mail: scott.sieburth@temple.edu
First published on 31st July 2014
Abstract
Polycyclic aromatics undergo [4 + 4] photocycloaddition to give substituted cyclooctadiene products. We have examined the [4 + 4] photoreactivity of these aromatics with enynes using a recently developed substitution pattern that undergoes a 1,3-hydrogen migration, forming a single cycloadduct. Several substrates yield stable, isomerized [4 + 4] adducts.
Higher-order cycloaddition reactions are powerful approaches for construction of molecular complexity.1,2 Photo-[4 + 4] dimerization of 1,3-dienes is, in principle, a direct way to access 1,5-cyclooctadienes 1, with up to four stereogenic centers, but the yield of this reaction is less than 1%, Scheme 1.3 Similarly, an enyne might undergo a [4 + 4] cycloaddition with a 1,3-diene to yield 1,2,5-cyclooctatriene 2. The product 2, unlike 1, is rare because of its strain.4 This strain is nicely illustrated by 1,2-cyclooctadiene 3, which undergoes a thermal [2 + 2] cycloaddition producing dimer 4.5
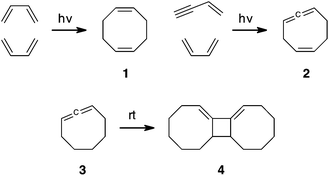 | ||
| Scheme 1 Cycloaddition of dienes and enynes can yield medium rings. Cycloocta-1,2-dienes are unstable. | ||
The use of 2-pyridones as [4 + 4] photocycloaddition partners has proven to be a general and efficient method for constructing densely functionalized 1,5-cyclooctadiene structures like 6, Scheme 2.6 Pyridones undergo [4 + 4] photocycloaddition not only with themselves, but with furan, naphthalene and 1,3-dienes.7 We have recently added 1,3-enynes to this list.8,9 The photocycloaddition of the tethered pyridone–enyne 7 efficiently yields adduct 8 but this compound is very short-lived. The isolated products are derived from dimerization of 8, structures analogous to the parent 1,2-cyclooctadiene dimer 4. This dimerization occurs with both of the allene double bonds, with more than a dozen products possible (representative examples have been characterized).9
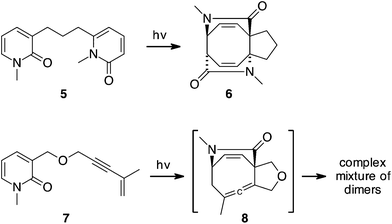 | ||
| Scheme 2 Photocycloaddition of pyridones with themselves and with enynes are efficient reactions but the latter yields unstable products. | ||
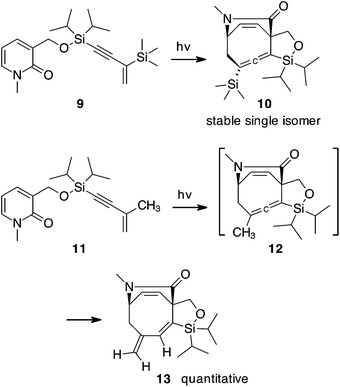
In more recent studies we have explored approaches to stabilize and/or trap the reactive allene photoproducts 8. Johnson demonstrated that a tert-butyl group attached to allene 3 results in a stable structure5 and we therefore have explored analogs of 8 designed to slow or prevent dimerization. This strategy led to the cycloadduct 10 with silanes on both ends of the allene, Scheme 3. Structure 10 appears to be indefinitely stable when stored cold, however, it remains unstable to chromatography.10 For photosubstrate 11, with diisopropylsilyl and methyl groups on the enyne, a rearrangement path to stability was discovered. Photocycloaddition of 11 presumably leads to cyclooctatriene 12. This allene is not observed, because it undergoes a rapid 1,3-hydrogen shift producing 1,3-diene 13 quantitatively. While still sensitive, this product can be readily isolated in 67% yield.10
After identifying this combination of methyl and diisopropylsilyl groups in 11 as an ideal enyne [4 + 4] participant that quantitatively yields a single product, we have explored the participation of this enyne unit with other unsaturated structures known to participate in [4 + 4] photocycloadditions, and report the results of those studies herein.
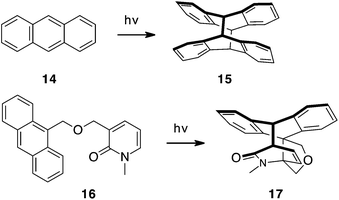
The first organic photoreaction, reported in 1867, was [4 + 4] photocycloaddition of anthracene 14 dimerizing to 15, Scheme 4.11 Both inter- and intramolecular photodimerization of anthracenes have been extensively studied.12–14 With these as precedent and inspiration, enyne–anthracene photocycloaddition was studied with inter- and intramolecular examples (Schemes 5 and 6, respectively). As a preamble to those studies we have investigated the photoreactivity of anthracene with 2-pyridones using 16 as a substrate, Scheme 4. Irradiation of this substrate with a medium-pressure mercury lamp for one hour gave quantitative conversion to adduct 17.
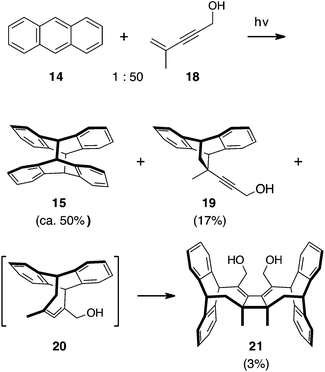 | ||
| Scheme 5 Intermolecular anthracene–enyne photoreaction yields a small amount of [4 + 4] cross adduct as the dimer. | ||
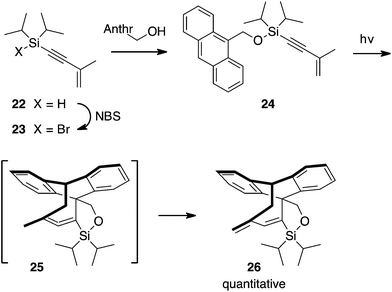 | ||
| Scheme 6 Anthracene undergoes a rapid and quantitative cycloaddition–isomerization reaction with the methyl enyne. | ||
The intermolecular photoreaction of enynes and anthracene 14 was first tested with 4-methyl-4-penten-2-yn-1-ol 18. Irradiation of mixture of 14 with 50 equivalents of enyne 18 in benzene at ambient temperature for 2 h gave dimerization of anthracene (15) as the major reaction path, isolated in 50% yield. An apparent [4 + 2] adduct 19 was isolated in 17% yield. Compound 21 was isolated in 3% yield. Product 21 results from [4 + 4] cycloaddition of the enyne with anthracene and the initially formed 20 undergoes a thermal [2 + 2] dimerization. The [4 + 2] adduct 19 could result from a thermal Diels–Alder reaction, however the short duration and low temperature of this reaction makes photo-[4 + 2] reaction a more likely pathway.15
Dimer 21 is a symmetric product (NMR, twenty 13C signals) with the cyclobutane formed from the methyl-substituted end of the allene. This identification is based, in part, on the chemical shift of the methyl group singlet for 21 that is consistent with its attachment to an aliphatic carbon rather than an alkene (1.89 ppm).8 While depicted as the cis isomer, the trans substituted cyclobutane isomer cannot be ruled out.
In contrast to the intermolecular reaction in which a [4 + 4] product was formed in low yield, the intramolecular reaction with the diisopropylsilyl ether gave a quantitative yield of the [4 + 4] adduct. Preparation of photosubstrate silyl ether 24 from 9-hydroxymethylanthracene began with oxidative bromination of silane 22 using NBS, to give 23, Scheme 6. Bromosilane 23, without isolation, was mixed with the alcohol and triethylamine to yield ether 24.
Irradiation of 24 using a Pyrex-filtered medium pressure mercury lamp for one hour cleanly converted 24 to the cycloaddition product 26. Analogous to our findings for cycloaddition with 2-pyridone 11, isomerization by 1,3-hydrogen shift was too fast to observe; compound 26 formed cleanly and was the only product formed.
A somewhat more complex reaction sequence is observed with the lower homolog naphthalene. Silyl ethers of both 1- and 2-naphthyl methanols 27 and 28 were prepared analogously to 24, Scheme 7. Irradiation of these substrates with a Pyrex-filtered medium-pressure mercury lamp did not result in productive photochemistry, nor did irradiation with Vycor-filtered light. When the 2-methoxy analog 29 was irradiated, however, a rapid photoreaction again was observed, quantitatively forming the cycloaddition – rearrangement product 31. Purification of this product by chromatography over silica gel did not yield 31 but instead gave the corresponding ketone 32.
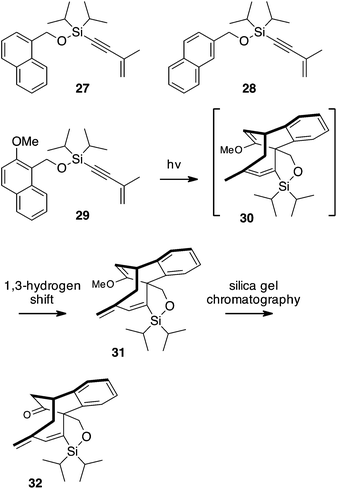 | ||
| Scheme 7 Methoxy-substituted naphthalene 29 undergoes a rapid and quantitative cycloaddition–isomerization reaction with the tethered enyne. Chromatography results in enol ether hydrolysis. | ||
The very facile hydrolysis of enol ether 31 is well precedented in the literature of naphthalene photodimerization reactions, and reflects the strain inherent in these [4 + 4] photoproducts.16
Following successful cycloadditions of the silicon-tethered enyne with anthracene and naphthalene, we turned our attention to the next lower homologue. Benzene has a rich photochemistry literature, with three primary modes of addition with alkenes and alkynes, so called ortho, meta and para cycloadditions.17 Among these, meta cycloaddition has been most widely studied. In reactions with 1,3-dienes only benzonitrile has been reported to yield [4 + 4] “para” photoadduct 34, Scheme 8. Photocycloaddition with 2,3-dimethyl-1,3-butadiene and with furan leads to adducts attached at the positions 2 and 5 of the benzonitrile.18,19 Coupling enyne 23 with the corresponding alcohols gave 35 and 36. Irradiation of these using a Rayonette photoreactor equipped with 254 nm lights, and with a medium-pressure mercury lamp – both Pyrex and Vycor filtered – failed to yield any cycloaddition products.
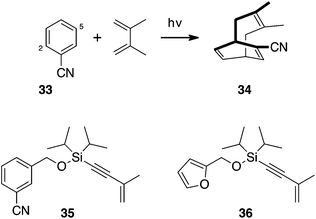 | ||
| Scheme 8 Benzonitrile undergoes [4 + 4] (aka “para”) cycloaddition with 1,3-dienes but neither 35 nor 36 photoadd to tethered enynes. | ||
In the work described here we have studied the use of enynes as [4 + 4] photocycloaddition participants with reactants other than 2-pyridones, our original vehicle. Comparing anthracene reactions with those of 2-pyridones, dimerization of the [4 + 4] products is a dominant path when the products are relatively unhindered. Intermolecular reactions form [4 + 4] adducts in low yield. Intramolecular reactions, in contrast, efficiently form [4 + 4] adducts between the enyne and the aromatic. When the [4 + 4] adducts of enynes are formed, dimerization of the resulting allenes is rapid unless steric shielding of the allene is substantial. A diisopropylsilyl group can provide enough shielding to allow for competing strain-relief paths to compete with the dimerization. In these examples, a 1,3-hydrogen migration leads to a single 1,3-diene product. This has allowed us to identify anthracene and naphthalenes as substrates for enyne [4 + 4] photocycloaddition chemistry, opening up new avenues for application of this cycloaddition process.
Acknowledgements
This work was supported by the National Science Foundation (1152159).Notes and references
- S. H. Bertz and T. J. Sommer, Chem. Commun., 1997, 2409 RSC.
- S. H. Bertz, New J. Chem., 2003, 27, 870 RSC.
- R. Srinivasan and F. I. Sonntag, J. Am. Chem. Soc., 1965, 87, 3778 CrossRef CAS.
- W. R. Roth and T. Bastigkeit, Liebigs Ann. Chem., 1996, 2171 CrossRef CAS PubMed.
- J. D. Price and R. P. Johnson, Tetrahedron Lett., 1986, 27, 4679 CrossRef CAS.
- S. McN. Sieburth, Photochemical reactivity of pyridones, in CRC Handbook of Organic Photochemistry and Photobiology, ed. W. Horspool and F. Lenci, CRC Press, Boca Raton, FL, 2004 Search PubMed.
- S. McN. Sieburth, K. F. McGee, F. Zhang and Y. Chen, J. Org. Chem., 2000, 65, 1972 CrossRef CAS.
- S. Kulyk, W. G. Dougherty Jr., W. S. Kassel, S. A. Fleming and S. McN. Sieburth, Org. Lett., 2010, 12, 3296 CrossRef CAS PubMed.
- S. Kulyk, W. G. Dougherty, W. S. Kassel, M. J. Zdilla and S. McN. Sieburth, Org. Lett., 2011, 13, 2180 CrossRef CAS PubMed.
- S. Kulyk, B. B. Khatri and S. McN. Sieburth, Org. Lett., 2014, 16 DOI:10.1021/ol501831a.
- J. Fritzsche, J. Prakt. Chem., 1867, 101, 333 CrossRef PubMed.
- J. J. McCullough, Chem. Rev., 1987, 87, 811 CrossRef CAS.
- H. D. Becker, Adv. Photochem., 1990, 15, 139 CAS.
- H. D. Becker, Chem. Rev., 1993, 93, 145 CrossRef CAS.
- J. P. Simons, Trans. Faraday Soc., 1960, 56, 391 RSC.
- T. Teitei, D. Wells, T. H. Spurling and W. H. F. Sasse, Aust. J. Chem., 1978, 31, 85 CrossRef CAS.
- U. Streit and C. G. Bochet, Beilstein J. Org. Chem., 2011, 7, 525 CrossRef CAS PubMed.
- K. Okumura, S. Takamuku and H. Sakurai, J. Soc. Chem. Ind., Jpn., 1969, 72, 200 CAS.
- A. Gilbert and O. Griffiths, J. Chem. Soc., Perkin Trans. 1, 1993, 1379 RSC.
Footnotes |
| † Dedicated to Professor Max Malacria on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures and spectra. See DOI: 10.1039/c4qo00190g |
| § Current address: Department of Chemistry, University of California at Berkeley, Berkeley, CA 94720. |
| This journal is © the Partner Organisations 2014 |