Efficient access to 1H-indazoles via copper-catalyzed cross-coupling/cyclization of 2-bromoaryl oxime acetates and amines†
Xiaodong
Tang
,
Hanling
Gao
,
Jidan
Yang
,
Wanqing
Wu
and
Huanfeng
Jiang
*
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, China. E-mail: jianghf@scut.edu.cn; cewuwq@scut.edu.cn; Fax: +86 20-87112906; Tel: +86 20-87112906
First published on 12th November 2014
Abstract
We describe a novel and useful method to provide 1H-indazoles via copper-catalyzed tandem reaction which is triggered by an Ullmann-type reaction and followed by N–N bond formation. Arylamines, alkylamines and sulfonamides could smoothly couple with 2-bromoaryl oxime acetates and various 1H-indazoles were formed in good to excellent yields under mild reaction conditions.
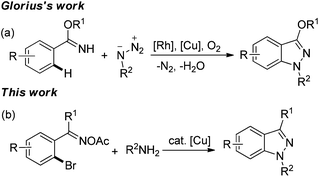
Due to a wide range of pharmaceutical activities, the 1H-indazole subunit has attracted much attention from synthetic chemists, and it has been widely used for anticancer, anti-inflammatory, anti-HIV, antifertility applications and in contraceptive drugs.1 Consequently, many methods have been developed for the construction of 1H-indazole frameworks, including classical diazotizations and nitrosation reactions,2 condensation of hydrazine with ortho-substituted benzaldehydes,3a,b [3 + 2] cycloaddition of arynes with diazo compounds or hydrazones3c–e and cyclization of arylamino oximes.3f,g With the development of transition metal catalysis, some transition metal-catalyzed routes to the 1H-indazole unit also have been realized. For example, Voskoboynikov et al. reported a palladium-catalyzed cyclization of arylhydrazones to form the 1H-indazole derivatives.4a Copper-catalyzed amination reactions were also used for 1H-indazole subunits.5 Olmo and coworkers have developed an efficient synthesis for indazoles via N-arylation of hydrazines, followed by intramolecular dehydration.5a However, most of these methods have some limitations, such as long reaction time, poor functional group tolerance, and low conversion. In addition, the biggest problem is the use of toxic organo-hydrazines. To the best of our knowledge, few examples of constructing 1H-indazoles via the formation of N–N bonds have been reported.3f,g,6 Recently, Glorius et al. described an efficient synthesis of 1H-indazoles from arylimidates and organo azides via RhIII/CuII-cocatalyzed C–H activation and C–N/N–N bond formations (Scheme 1a).6 For this we propose a method which utilizes the N–N bond formation to obtain the 1H-indazole unit only using a cheap metal (copper) as the catalyst and without using carcinogenic hydrazines, which is desired and challenging.
Copper-mediated Ullmann-type reaction was discovered a century ago.7 However, it has not been fully utilized due to high reaction temperature, limitations of the substrates, and need of stoichiometric copper salts. In recent years, great breakthroughs have been achieved by some research groups,8 which made Ullmann-type reactions feasible with a catalytic amount of copper salts and low temperature. These breakthroughs also made the Ullmann-type reaction a good method to construct C–C, C–O and C–N bonds. In the last several years, oxime esters have been used for the nitrogen-containing heterocycles, such as pyridines,9 pyrroles,10 and imidazo[1,2-a]pyridines11 in the presence of copper salts. Based on our previous work on oxime esters10a,11,12 and the development of Ullmann-type reactions, we envisioned that we could obtain nitrogen-containing heterocycles via tandem reaction which is triggered by Ullmann-type reactions and then undergo N–N bond formation using oxime acetates not only as substrates but also as internal oxidants. Herein, we disclose a novel and efficient strategy for 1H-indazoles from 2-bromoaryl oxime acetates and amines via copper-catalyzed tandem reaction involving a sequential Ullmann-type reaction and a N–N bond formation process (Scheme 1b).
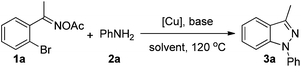
Initially, the transformation of 1-(2-bromophenyl)ethanone oxime acetate (1a) and aniline (2a) was taken as the model system to screen reaction parameters (Table 1). To our delight, product 3a could be obtained in 71% GC yield when we utilized CuBr (10 mol%) as a catalyst and K2CO3 as a base in DMSO at 120 °C under a N2 atmosphere after 6 h (Table 1, entry 1). Different copper salts such as CuI, CuCl, Cu(OAc)2, and Cu(OTf)2 were also examined in this process (entries 2–5) and CuCl proved to be the best catalyst, affording product 3a in 86% isolated yield. No product could be observed without the copper catalyst (entry 6). The investigation of different bases, including Cs2CO3, Na2CO3, NaHCO3, NaHSO3, and Et3N, indicated that K2CO3 was the best choice (entries 7–11). The yield decreased to 18% in the absence of a base (entry 12). Decreasing the temperature to 100 °C, the yield sharply decreased to 53% and the reason was that the Ullmann-type reaction could not proceed smoothly at lower temperature (entry 13). Different solvents such as toluene, DMF, DMA, NMP and MeCN were screened. Except acetonitrile, which gave 85% yield, other solvents were not good for this reaction (entries 14–18). Thus, the optimal reaction conditions were 1a (0.5 mmol), 2a (0.6 mmol), CuCl (10 mol%), and K2CO3 (1.0 mmol), in 2 mL DMSO at 120 °C under a N2 atmosphere for 6 h.
| Entry | [Cu] | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: unless otherwise noted, all reactions were performed with 1a (0.5 mmol), 2a (0.6 mmol), a catalyst (10 mol%), a base (1 mmol) and a solvent (2 mL) at 120 °C under a N2 atmosphere for 6 h. b Determined by GC based on 1a. c The reaction at 100 °C. | ||||
| 1 | CuBr | K2CO3 | DMSO | 71 |
| 2 | CuI | K2CO3 | DMSO | 86 |
| 3 | CuCl | K2CO3 | DMSO | 92 (86) |
| 4 | Cu(OTf)2 | K2CO3 | DMSO | 11 |
| 5 | Cu(OAc)2 | K2CO3 | DMSO | 60 |
| 6 | — | K2CO3 | DMSO | 0 |
| 7 | CuCl | Cs2CO3 | DMSO | 63 |
| 8 | CuCl | Na2CO3 | DMSO | 33 |
| 9 | CuCl | NaHCO3 | DMSO | 42 |
| 10 | CuCl | NaHSO3 | DMSO | 11 |
| 11 | CuCl | NEt3 | DMSO | 6 |
| 12 | CuCl | — | DMSO | 18 |
| 13c | CuCl | K2CO3 | DMSO | 53 |
| 14 | CuCl | K2CO3 | Toluene | 8 |
| 15 | CuCl | K2CO3 | DMF | 41 |
| 16 | CuCl | K2CO3 | DMA | 69 |
| 17 | CuCl | K2CO3 | NMP | 37 |
| 18 | CuCl | K2CO3 | MeCN | 85 |
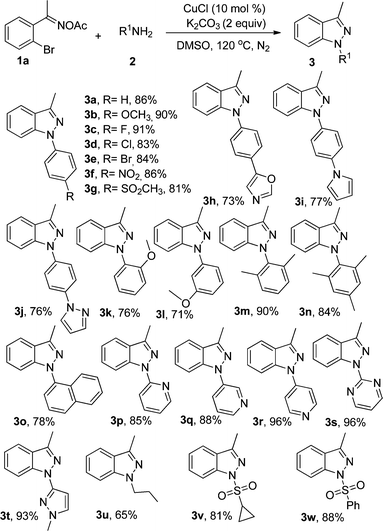
With the optimum reaction conditions in hand, we started to investigate the scope of amines (Table 2). Various functional groups including methoxyl, fluoro, chloro, bromo, nitro, and methylsulfonyl could be tolerated at the para-position of aniline and the desired 1H-indazoles 3a–3g were formed in good to excellent yields. 4-Heterocyclic-substituted anilines, such as 4-(oxazol-5-yl)aniline, 4-(1H-pyrrol-1-yl)aniline, and 4-(1H-pyrazol-1-yl)aniline, were also suitable substrates to afford the corresponding 1H-indazoles (3h–3j). When 2-methoxyaniline, 3-methoxyaniline, 2,6-dimethylaniline and 2,4,6-trimethylaniline were subjected to the reaction system, 3k–3n could be isolated in 76%, 71%, 90% and 84% yields, respectively. In addition, other aromatic or heterocyclic amines including the pyridine ring, which are not usually applicable in copper-catalyzed reactions, could also be transformed to the target products in yields ranging from 78% to 96% (3o–3t). It was exciting that the alkyl amines were good starting materials and the corresponding products could be generated in moderate yields (3u). It was worth mentioning that sulfonamide derivatives could transform into products in good yields, which gave a useful sulfone functional group (3v–3w).
| a The reactions were carried out at 120 °C, using 1a (0.5 mmol), 2 (0.6 mmol), CuCl (10 mol%), and K2CO3 (1.0 mmol), in DMSO (2 mL) under a N2 atmosphere for 6 h. Yields refer to isolated yields. |
|---|
|
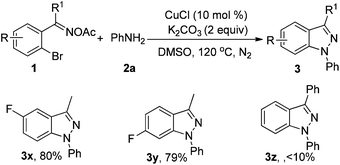
Subsequently, we examined various oxime acetates shown in Table 3. 2-Bromoaryl oxime acetates such as 1-(2-bromo-4-fluorophenyl)ethanone oxime acetate and 1-(2-bromo-5-fluorophenyl)ethanone oxime acetate also reacted well with aniline to afford the corresponding products (3x–3y) in 80% and 79% yields, respectively. However, when (2-bromophenyl)(phenyl)methanone oxime acetate was used as the substrate, the corresponding product 3z was obtained in lower yield and the 3-phenylbenzo[d]isoxazole was formed via (2-bromophenyl)(phenyl)methanone oxime acetate turning into (2-bromophenyl)(phenyl)methanone oxime and then the intramolecular Ullmann-type reaction.13a
| a The reactions were carried out at 120 °C, using 1 (0.5 mmol), 2a (0.6 mmol), CuCl (10 mol%), and K2CO3 (1.0 mmol), in DMSO (2 mL) under a N2 atmosphere for 6 h. Yields refer to isolated yields. |
|---|
|
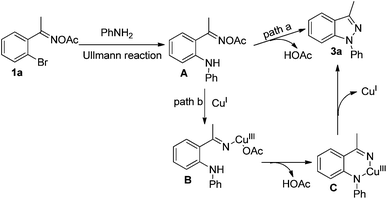
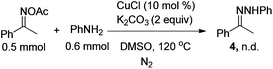
A control experiment was conducted to gain more insight into the mechanism. When we coupled acetophenone oxime acetate with aniline under the standard conditions, product 4 could not be obtained [eqn (1)], and the analogue of 1a easily went through the Ullmann-type reaction,13g suggesting that this reaction should be triggered by the Ullmann-type reaction. Based on this experiment and previous reports,13–16 a plausible mechanism for the present reaction is described in Scheme 2. Firstly, 1a was coupled with aniline to form intermediate Avia the copper-catalyzed Ullmann-type reaction.13 Then intermediate A might go through two possible pathways for the observed product. In path a, the amino group attacked the oxime acetate to form the desired 1H-indazole product 3a by releasing a molecule of HOAc.3f,g,14 The other pathway might go through an organocopper(III) process (path b). Oxidative addition of CuI to the N–O bond gave intermediate B.15 Subsequently, intermediate C was formed via the coordination of the nitrogen atom to copper(III), which simultaneously produced a molecule of HOAc which was neutralized by the base. Finally, intermediate C could be transferred to the desired product via reductive elimination (path b).16
 | (1) |
In conclusion, we have developed a novel and useful method for the construction of 1H-indazoles. This transformation is supposed to be triggered by the Ullmann-type reaction and then undergoes the N–N bond formation process. Various arylamines, alkylamines and sulfonamides could be applied to this reaction system and the desired 1H-indazole products were formed in good to excellent yields. In this process, the oxime acetates were not only used as substrates but also as internal oxidants. Moreover, the use of a catalytic amount of copper salts and no need for additional ligands makes this method attractive and practical. Further studies on the reaction scope and mechanism are currently in progress in our laboratory.
The authors thank the National Natural Science Foundation of China (21172076), the National Basic Research Program of China (973 Program) (2011CB808600), the Guangdong Natural Science Foundation (10351064101000000), and the Fundamental Research Funds for the Central Universities (2014ZP0004 and 2014ZZ0046) for financial support.
Notes and references
- (a) J. A. May, A. P. Dantanarayana, P. W. Zinke, M. A. McLaughlin and N. A. Sharif, J. Med. Chem., 2006, 49, 318 CrossRef CAS PubMed; (b) S. K. V. Vernekar, H. Y. Hallaq and G. Clarkson, J. Med. Chem., 2010, 53, 2324 CrossRef CAS PubMed; (c) D. E. N. Jacquot and M. Zollinger, Bioorg. Med. Chem. Lett., 2011, 21, 6297 CrossRef PubMed.
- T. Eicher and S. Hauptmann, The Chemistry of Heterocycles, Wiley-VCH, Weinheim, 2003 Search PubMed.
- (a) K. Lukin, M. C. Hsu, D. Fernando and M. R. Leanna, J. Org. Chem., 2006, 71, 8166 CrossRef CAS PubMed; (b) S. Caron and E. Vazquez, Synthesis, 1999, 588 CrossRef CAS PubMed; (c) T. Jin and Y. Yamamoto, Angew. Chem., Int. Ed., 2003, 42, 2681 CrossRef PubMed; (d) P. Li, C. Wu, J. J. Zhao, D. C. Rogness and F. Shi, J. Org. Chem., 2012, 77, 3149 CrossRef CAS PubMed; (e) Z. J. Liu, F. Shi, P. D. G. Martinez, C. Raminelli and R. C. Larock, J. Org. Chem., 2008, 73, 219 CrossRef CAS PubMed; (f) B. C. Wray and J. P. Stambuli, Org. Lett., 2010, 12, 4576 CrossRef CAS PubMed; (g) C. M. Counceller, C. C. Eichman, B. C. Wray and J. P. Stambuli, Org. Lett., 2008, 10, 1021 CrossRef CAS PubMed.
- (a) A. Y. Lebedev, A. S. Khartulyari and A. Z. Voskoboynikov, J. Org. Chem., 2005, 70, 596 CrossRef CAS PubMed; (b) K. Inamoto, M. Katsuno, T. Yoshino, Y. Arai, K. Hiroya and T. Sakamoto, Tetrahedron, 2007, 44, 5664 Search PubMed; (c) J. J. Song and N. K. Yee, Tetrahedron Lett., 2001, 42, 2937 CrossRef CAS; (d) C. S. Cho, D. K. Lim, N. H. Heo, T. Kimb and S. C. Shim, Chem. Commun., 2004, 104 RSC; (e) V. Lefebvre, T. Cailly, F. Fabis and S. Rault, J. Org. Chem., 2010, 75, 2730 CrossRef CAS PubMed.
- (a) D. Vina, E. D. Olmo, J. L. López-Pérez and A. S. Feliciano, Org. Lett., 2007, 9, 525 CrossRef CAS PubMed; (b) X. D. Xiong, Y. W. Jiang and D. W. Ma, Org. Lett., 2012, 14, 2552 CrossRef CAS PubMed; (c) K. C. Washizuka, S. Minakata, I. Ryu and M. Komatsu, Tetrahedron Lett., 2005, 46, 7553 CrossRef PubMed.
- D. Yu, M. Suri and F. Glorius, J. Am. Chem. Soc., 2013, 135, 8802 CrossRef CAS PubMed.
- (a) F. Ullmann, Ber. Dtsch. Chem. Ges., 1903, 36, 2382 CrossRef; (b) F. Ullmann, Ber. Dtsch. Chem. Ges., 1904, 37, 853 CrossRef; (c) I. Goldberg, Ber. Dtsch. Chem. Ges., 1906, 39, 1691 CrossRef.
- (a) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 CrossRef CAS PubMed; (b) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS PubMed; (c) D. W. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 852 CrossRef PubMed.
- (a) S. Liu and L. S. Liebeskind, J. Am. Chem. Soc., 2008, 130, 6918 CrossRef CAS PubMed; (b) Z. Ren, Z. Zhang, B. Yang, Y. Wang and Z. Guan, Org. Lett., 2011, 13, 5394 CrossRef CAS PubMed; (c) Y. Wei and N. Yoshikai, J. Am. Chem. Soc., 2013, 135, 3756 CrossRef CAS PubMed.
- (a) X. Tang, L. Huang, C. Qi, W. Wu and H. Jiang, Chem. Commun., 2013, 49, 9597 RSC; (b) L. Ran, Z. Ren, Y. Wang and Z. Guan, Green Chem., 2014, 16, 112 RSC.
- H. Huang, X. Ji, X. Tang, M. Zhang, X. Li and H. Jiang, Org. Lett., 2013, 15, 6254 CrossRef CAS PubMed.
- X. Tang, L. Huang, Y. Xu, J. Yang, W. Wu and H. Jiang, Angew. Chem., Int. Ed., 2014, 53, 4205 CrossRef CAS PubMed.
- (a) P. De, N. Nonappa, K. Pandurangan, U. Maitra and S. Wailes, Org. Lett., 2007, 9, 2767 CrossRef CAS PubMed; (b) H. Rao, H. Fu, Y. Jiang and Y. Zhao, J. Org. Chem., 2005, 70, 8107 CrossRef CAS PubMed; (c) L. Rout, S. Jammi and T. Punniyamurthy, Org. Lett., 2010, 12, 5688 CrossRef PubMed; (d) H. Rao, Y. Jin, H. Fu, Y. Jiang and Y. Zhao, Chem. – Eur. J., 2006, 12, 3636 CrossRef CAS PubMed; (e) J. Jiao, X. Zhang, N. Chang, J. Wang, J. Wei, X. Shi and Z. Chen, J. Org. Chem., 2011, 76, 1180 CrossRef CAS PubMed; (f) H. Xu and C. Wolf, Chem. Commun., 2009, 1715 RSC; (g) R. Xie, H. Fu and Y. Ling, Chem. Commun., 2011, 47, 8976 RSC.
- (a) A. Hassner and M. J. Michelson, J. Org. Chem., 1962, 27, 298 CrossRef CAS; (b) M. J. Campbell and J. S. Johnson, Org. Lett., 2007, 9, 1521 CrossRef CAS PubMed.
- (a) S. Liu, Y. Yu and L. S. Liebeskind, Org. Lett., 2007, 9, 1947 CrossRef CAS PubMed; (b) A. John and K. M. Nicholas, Organometallics, 2012, 31, 7914 CrossRef CAS.
- (a) J. J. Neumann, M. Suri and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 7790 CrossRef CAS PubMed; (b) S. Ueda and H. Nagasawa, J. Am. Chem. Soc., 2009, 131, 15080 CrossRef CAS PubMed; (c) M. Suri, T. Jousseaume, J. J. Neumann and F. Glorius, Green Chem., 2012, 14, 2193 RSC; (d) Z. Chen, Q. Yan, Z. Liu, Y. Xu and Y. Zhang, Angew. Chem., Int. Ed., 2013, 52, 13324 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, characterization of all compounds, copies of 1H and 13C NMR spectra for selected compounds. See DOI: 10.1039/c4qo00244j |
| This journal is © the Partner Organisations 2014 |