Facile solid-phase synthesis of PNA–peptide conjugates using pNZ-protected PNA monomers†
Abstract
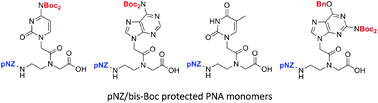
PNA–peptide conjugates are useful molecular tools in chemical biology and biotechnology. Although several approaches have been developed to synthesize PNA–peptide conjugates, more efficient methods are still needed. In this report a new pNZ (p-nitrobenzyloxycarbonyl)/bis-Boc strategy was developed as an alternative backbone/nucleobase protecting group method. The mild deprotection conditions of pNZ group and pNZ's full orthogonality with Fmoc solid-phase synthesis enable a new dimension of synthetic flexibility and practicality to generate versatile PNA–peptide conjugates.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers for 2014

 Please wait while we load your content...
Please wait while we load your content...