Themed collection Catalysis & biocatalysis in OBC

Modern metal-catalyzed and organocatalytic methods for synthesis of coumarin derivatives: a review
Organocatalytic methods and light-mediated methods have proved to be effective in chemical synthetic science. This paper compares them with the more established metal-catalyzed methods for the synthesis of a pharmaceutical core: coumarin.
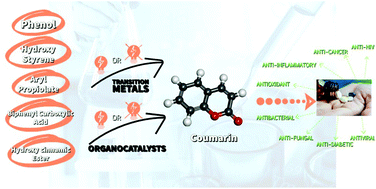
Org. Biomol. Chem., 2022,20, 4846-4883
https://doi.org/10.1039/D2OB00491G
Metal–ligand cooperative approaches in homogeneous catalysis using transition metal complex catalysts of redox noninnocent ligands
Catalysis offers a straightforward route to prepare various value-added molecules starting from readily available raw materials.

Org. Biomol. Chem., 2022,20, 296-328
https://doi.org/10.1039/D1OB01153G
Recent developments in the synthesis of nitrogen-containing heterocycles from β-aminovinyl esters/ketones as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N donors
C–N donors
The synthesis of nitrogen-containing heterocycles from β-aminovinyl esters(ketones) was reviewed according to the classification of the final heterocycle synthesis.
![Graphical abstract: Recent developments in the synthesis of nitrogen-containing heterocycles from β-aminovinyl esters/ketones as C [[double bond, length as m-dash]] C–N donors](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1OB01998H&imageInfo.ImageIdentifier.Year=2022)
Org. Biomol. Chem., 2022,20, 282-295
https://doi.org/10.1039/D1OB01998H
Deoxygenation reactions in organic synthesis catalyzed by dioxomolybdenum(VI) complexes
This review summarizes the recent advances in deoxygenation reactions of S–O, N–O and C–O bonds catalyzed by dioxomolybdenum(VI) complexes.
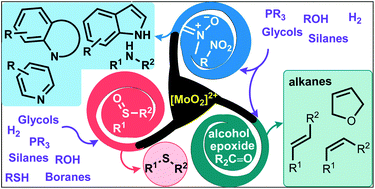
Org. Biomol. Chem., 2021,19, 10472-10492
https://doi.org/10.1039/D1OB01939B
Recent advances in the organocatalytic applications of cyclopropene- and cyclopropenium-based small molecules
A comprehensive discussion on the organocatalytic applications of cyclopropene-based small molecules such as cyclopropenium salts, cyclopropenimines, cyclopropenylidenes, and cyclopropenones in organic transformations is reported.
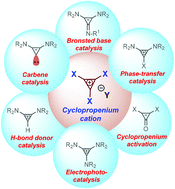
Org. Biomol. Chem., 2021,19, 9541-9564
https://doi.org/10.1039/D1OB01549D
Heteroleptic dirhodium(II,II) paddlewheel complexes as carbene transfer catalysts
This review highlights the applications of dirhodium(II,II) paddlewheel complexes with a heteroleptic scaffold.
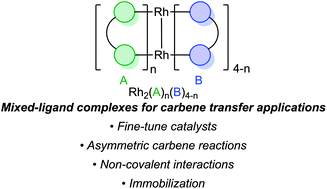
Org. Biomol. Chem., 2021,19, 8886-8905
https://doi.org/10.1039/D1OB01414E
Recent advances in rhodium-catalyzed C(sp2)–H (hetero)arylation
Recent achievements in rhodium-catalyzed arylations through C(sp2)–H bond activation were summarized.

Org. Biomol. Chem., 2021,19, 8442-8465
https://doi.org/10.1039/D1OB01190A
Transition-metal-catalyzed C–H bond activation/functionalization and annulation of phthalazinones
Heteroatoms (O and N) on the phthalazinones are exploited for diverse functionalization with high regioselectivity by the intervention of transition-metal catalysts like Rh, Ru, Pd, & Ir and, thus augmenting its value.
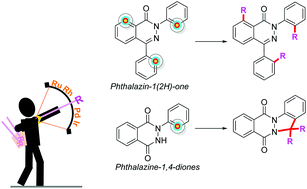
Org. Biomol. Chem., 2021,19, 8165-8183
https://doi.org/10.1039/D1OB01616D
Modern methods for the synthesis of perfluoroalkylated aromatics
This review summarizes the most recent organometallic, photochemical and electrochemical methods for the synthesis of perfluoroalkylated aromatic scaffolds, a family of compounds that is omnipresent in modern commercial and research applications.
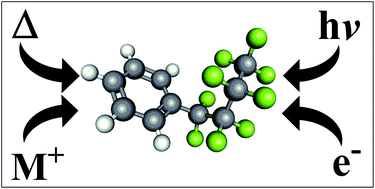
Org. Biomol. Chem., 2021,19, 7116-7128
https://doi.org/10.1039/D1OB01122G
Carbon dioxide based methodologies for the synthesis of fine chemicals
Rapid environmental changes triggered by the increase in the concentration of heat-absorbing gases such as CO2 in the atmosphere have become a major cause of concern.
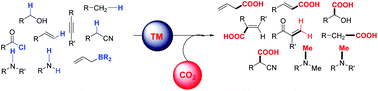
Org. Biomol. Chem., 2021,19, 5725-5757
https://doi.org/10.1039/D1OB00755F
Iminosugars as glycosyltransferase inhibitors
The review describes the syntheses and inhibition properties of pyrrolidine, piperidine, azepane, pyrrolizidine and indolizidine iminosugars, as well as iminosugar nucleotides and iminodi- and -oligosaccharides.
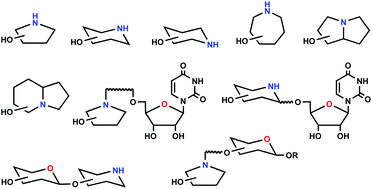
Org. Biomol. Chem., 2021,19, 5439-5475
https://doi.org/10.1039/D1OB00382H
Thioxanthone: a powerful photocatalyst for organic reactions
Thioxanthone is a powerful photocatalyst. In this review, its photophysical properties and its applications in photopolymerisation and in organic synthesis are highlighted.

Org. Biomol. Chem., 2021,19, 5237-5253
https://doi.org/10.1039/D1OB00221J
Phosphorus containing porous organic polymers: synthetic techniques and applications in organic synthesis and catalysis
This review features the recent advancement made in phosphorus-containing POPs, highlighting different synthetic techniques for their preparation and application in organic synthesis.

Org. Biomol. Chem., 2021,19, 4174-4192
https://doi.org/10.1039/D1OB00137J
Recent advances in nickel-catalyzed C–C and C–N bond formation via HA and ADC reactions
In this review article, recent advances in nickel-catalyzed hydrogen auto-transfer (HA) and acceptorless dehydrogenative coupling (ADC) reactions for the construction of C–C and C–N bonds have been discussed.
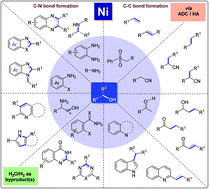
Org. Biomol. Chem., 2021,19, 4213-4227
https://doi.org/10.1039/D1OB00080B
Recent advances in the copper-catalyzed aerobic Csp3–H oxidation strategy
This mini-review aims to cover the Cu-catalyzed aerobic benzylic and α-carbonyl Csp3–H oxidations and that of the carbon next to an amine group in the past five years.
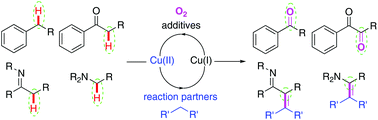
Org. Biomol. Chem., 2021,19, 3569-3583
https://doi.org/10.1039/D1OB00081K
Metallaphotoredox catalysis with organic dyes
Here…comes the fun…Combination of metals and organic photocatalysts allows the practical invention of new methodologies!
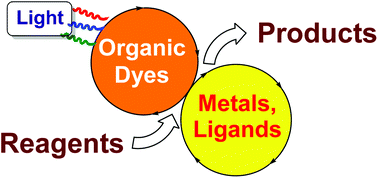
Org. Biomol. Chem., 2021,19, 3527-3550
https://doi.org/10.1039/D1OB00196E
α,β-Unsaturated acyl ammonium species as reactive intermediates in organocatalysis: an update
α,β-Unsaturated acyl ammonium species are versatile intermediates that have been applied in a variety of transformations including Michael additions, domino reactions and cycloadditions.
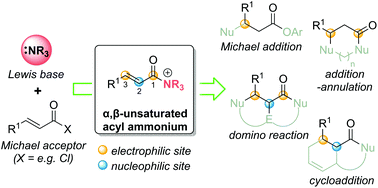
Org. Biomol. Chem., 2021,19, 2366-2384
https://doi.org/10.1039/D0OB02208J
A comprehensive insight into aldehyde deformylation: mechanistic implications from biology and chemistry
Aldehyde deformylation is one of the useful reactions in biology and organic syntheses and this review provides mechanistic insights into the same.
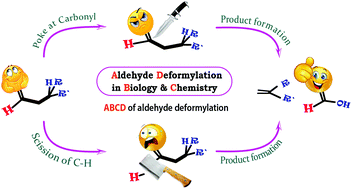
Org. Biomol. Chem., 2021,19, 1879-1899
https://doi.org/10.1039/D0OB02204G
Recent advances in Rh(III)/Ir(III)-catalyzed C–H functionalization/annulation via carbene migratory insertion
The review highlighted diverse annulations, including nitrogen, oxygen, sulfur heterocycles and carbocylizations via Rh(III)/Ir(III)-catalyzed C–H functionalization/annulation with various arene and carbene precursors.

Org. Biomol. Chem., 2021,19, 1438-1458
https://doi.org/10.1039/D0OB02309D
Biocatalytic approaches for enantio and diastereoselective synthesis of chiral β-nitroalcohols
Chiral β-nitroalcohols are versatile synthetic intermediates for several pharmaceuticals, and bioactive molecules. This review describes the importance and various biocatalytic approaches for their enantio and diastereoselective synthesis.
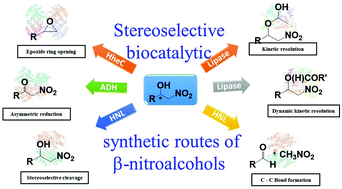
Org. Biomol. Chem., 2021,19, 322-337
https://doi.org/10.1039/D0OB02019B
Recent advances in using 4DPAIPN in photocatalytic transformations
1,3-Dicyano-2,4,5,6-tetrakis(diphenylamino)-benzene has emerged as a powerful and attractive metal-free organophotocatalyst for organic transformation and is expected to contribute to a great extent toward the advancement and development of synthetic methodologies.
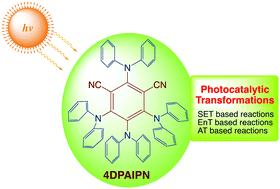
Org. Biomol. Chem., 2021,19, 313-321
https://doi.org/10.1039/D0OB01884H
Integrating abiotic chemical catalysis and enzymatic catalysis in living cells
We review hybrid systems of abiotic catalysis and enzymatic catalysis, which function in living cells. This research direction will stimulate multidisciplinary fields, including complex molecule synthesis, energy production, and life science.
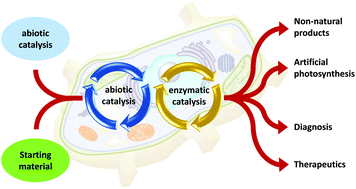
Org. Biomol. Chem., 2021,19, 37-45
https://doi.org/10.1039/D0OB01898H
Recent developments in the synthesis and applications of chiral ferrocene ligands and organocatalysts in asymmetric catalysis
This review highlights recent advances in the synthesis and application of ferrocene-containing chiral ligands and organocatalysts in asymmetric catalysis.
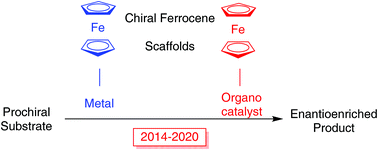
Org. Biomol. Chem., 2020,18, 9329-9370
https://doi.org/10.1039/D0OB01933J
Transition metal catalyzed glycosylation reactions – an overview
An overview of transition metal catalyzed glycosylation reactions is presented and the main trends in reactivity are discussed.
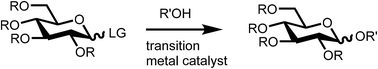
Org. Biomol. Chem., 2020,18, 9160-9180
https://doi.org/10.1039/D0OB01782E
Copper-catalysed radical reactions of alkenes, alkynes and cyclopropanes with N–F reagents
The mild generation of nitrogen-centred radicals from N–F reagents using copper-based catalysts has become a convenient synthetic tool.
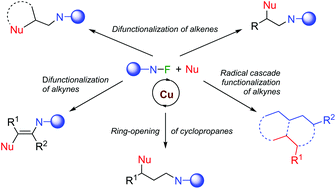
Org. Biomol. Chem., 2020,18, 8757-8770
https://doi.org/10.1039/D0OB01743D
Catalytic enantioselective synthesis using carbon dioxide as a C1 synthon
This review summarizes the advances in catalytic enantioselective reactions using CO2 as a C1 synthon, introduces strategies and discusses advantages and limitations, highlights the application, and outlines the synthetic opportunities.
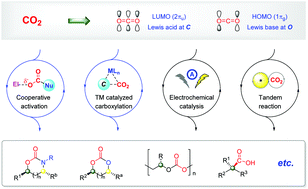
Org. Biomol. Chem., 2020,18, 8597-8619
https://doi.org/10.1039/D0OB01905D
Photo-induced 1,2-carbohalofunctionalization of C–C multiple bonds via ATRA pathway
Carbohalofunctionalization of C–C multiple bonds via atom transfer radical processes constitutes an efficient method for the construction of halogenated building blocks with complete atom economy. This review summarizes the recent advancements.
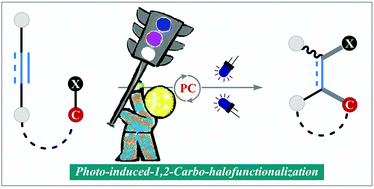
Org. Biomol. Chem., 2020,18, 8278-8293
https://doi.org/10.1039/D0OB01454K
Organic synthesis of high added value molecules with MOF catalysts
Recent examples of organic synthesis of fine chemicals and pharmaceuticals in confined spaces of MOFs are highlighted and compared with silica-based ordered porous solids, such as zeolites or mesoporous (organo)silica.
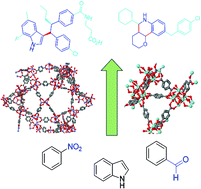
Org. Biomol. Chem., 2020,18, 8058-8073
https://doi.org/10.1039/D0OB01571G
Molecular crowding and RNA catalysis
Molecular crowding promotes RNA folding and catalysis and could have played vital roles in the evolution of primordial ribozymes and protocells.
Org. Biomol. Chem., 2020,18, 7724-7739
https://doi.org/10.1039/D0OB01695K
Recent advances in catalytic enantioselective multicomponent reactions
Multicomponent reactions have demonstrated a remarkable impact on the synthesis of complex compounds, with high atom economy. In this review, the last decade contributions to enantioselective MCRs by focusing on catalytic approaches are discussed.
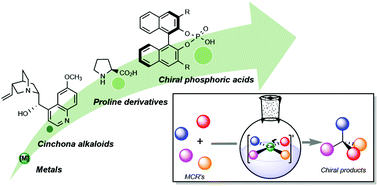
Org. Biomol. Chem., 2020,18, 7751-7773
https://doi.org/10.1039/D0OB01631D
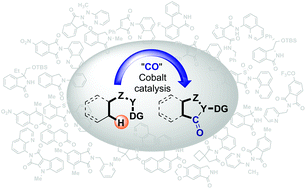
Cobalt-catalyzed carbonylation of the C–H bond
Direct carbonylation of the C–H bond is a great tool for installing a carbonyl group in a wide variety of substrates.
Org. Biomol. Chem., 2020,18, 7460-7466
https://doi.org/10.1039/D0OB01633K
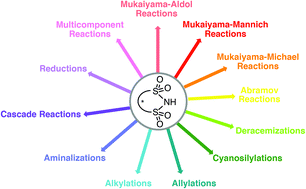
Chiral disulfonimides: a versatile template for asymmetric catalysis
Chiral disulfonimides are a powerful class of organocatalysts that are amenable to an impressive scope of reaction types. This review provides a complete analysis of their synthesis, successful applications, mechanistic insights, and unmet challenges.
Org. Biomol. Chem., 2020,18, 7485-7513
https://doi.org/10.1039/D0OB01742F
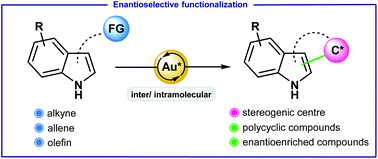
Gold-catalyzed enantioselective functionalization of indoles
This review documents the potential of enantioselective gold catalysis for the functionalization of indoles.
Org. Biomol. Chem., 2020,18, 6006-6017
https://doi.org/10.1039/D0OB01245A
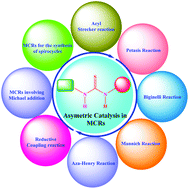
Recent applications of thiourea-based organocatalysts in asymmetric multicomponent reactions (AMCRs)
This review describes the recent applications of thiourea-based organocatalysts in asymmetric multicomponent reactions.
Org. Biomol. Chem., 2020,18, 5513-5532
https://doi.org/10.1039/D0OB00595A
Asymmetric copper-catalyzed conjugate additions of organometallic reagents in the syntheses of natural compounds and pharmaceuticals
Stereoselective copper-catalyzed conjugate additions of organometallic reagents serve as a versatile tool in the total syntheses of diverse natural compounds and pharmaceutical agents.
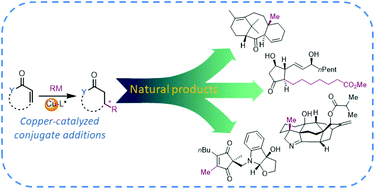
Org. Biomol. Chem., 2020,18, 3780-3796
https://doi.org/10.1039/D0OB00278J
Catalytic enantioselective decarboxylative nucleophilic addition reactions using chiral organocatalysts
Catalytic decarboxylative reactions are attractive as biomimetic and environmentally friendly reaction processes. This review summarizes recent results for organocatalytic enantioselective decarboxylative reactions from October 2013 to December 2019.
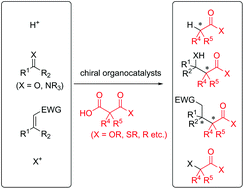
Org. Biomol. Chem., 2020,18, 2781-2792
https://doi.org/10.1039/D0OB00127A
Tandem transformations and multicomponent reactions utilizing alcohols following dehydrogenation strategy
In this review, the progress of tandem transformation of nitro, nitrile and azide functionalities is summarised to develop new C–C and C–N bonds as well as multi-component reactions using alcohols.
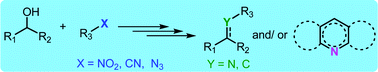
Org. Biomol. Chem., 2020,18, 2193-2214
https://doi.org/10.1039/C9OB02760B
Catalytic strategies towards 1,3-polyol synthesis by enantioselective cascades creating multiple alcohol functions
This review highlights the different enantioselective catalyst-controlled cascades creating multiple alcohol functions and ensuring through subsequent simple derivatization, the rapid preparation of extended 1,3-polyols.

Org. Biomol. Chem., 2020,18, 1025-1035
https://doi.org/10.1039/C9OB02675D
Recent trends in catalytic sp3 C–H functionalization of heterocycles
In this mini-review, we attempt to highlight gaps in existing techniques for sp3 C–H activation adjacent to heterocycles.
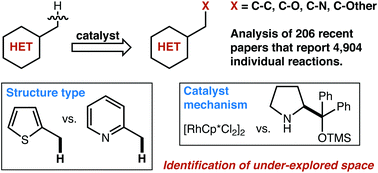
Org. Biomol. Chem., 2020,18, 606-617
https://doi.org/10.1039/C9OB01559K
Recent applications of chiral phosphoric acids in palladium catalysis
A variety of catalytic asymmetric reactions have been realized during the past decade through the combined action of palladium and chiral phosphoric acids (CPAs). This review surveys key examples and examines the underlying mechanisms of stereoinduction.
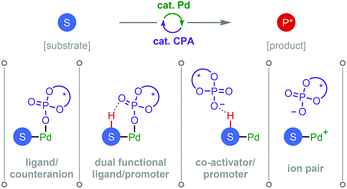
Org. Biomol. Chem., 2020,18, 618-637
https://doi.org/10.1039/C9OB02205H
Recent advances in cobalt-catalysed C–H functionalizations
Ready availability, low cost and low toxicity of cobalt salts have redirected the attention of researchers away from noble metals, such as Pd, Rh, and Ir, towards Co in the field of C–H functionalisation.
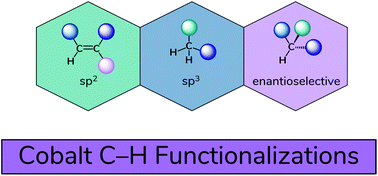
Org. Biomol. Chem., 2019,17, 10119-10141
https://doi.org/10.1039/C9OB01994D
Recent advances in modified TiO2 for photo-induced organic synthesis
The recent advancements of modified TiO2 materials as photocatalysts for organic synthesis are summarized.

Org. Biomol. Chem., 2019,17, 9977-9989
https://doi.org/10.1039/C9OB01739A
Catalytic enantioselective cross dehydrogenative coupling of sp3 C–H of heterocycles
The recent developments in the asymmetric functionalization of heterocycles via the catalytic enantioselective cross dehydrogenative coupling reactions of heterocyclic sp3 C–H bonds are highlighted in this review.

Org. Biomol. Chem., 2019,17, 9683-9692
https://doi.org/10.1039/C9OB02113B
G-quadruplexes as versatile scaffolds for catalysis
This review summarizes the beginning, progress, and prospects of non-canonical DNA-based hybrid catalysts focusing on G-quadruplexes as versatile scaffolds for catalysis.
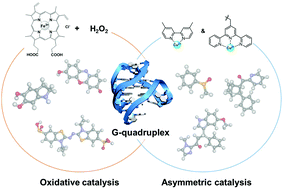
Org. Biomol. Chem., 2019,17, 9547-9561
https://doi.org/10.1039/C9OB01876J
Advances in asymmetric visible-light photocatalysis, 2015–2019
Asymmetric visible-light photocatalysis has recently drawn considerable attention of the scientific community owing to its unique activation modes and significance for the enantioselective green synthesis.
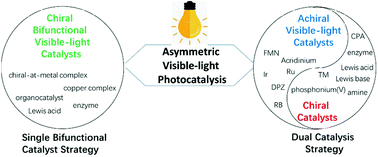
Org. Biomol. Chem., 2019,17, 8673-8689
https://doi.org/10.1039/C9OB01609K
Hetero-bimetallic cooperative catalysis for the synthesis of heteroarenes
Review covering the synthesis of 5- and 6-membered as well as condensed heteroarenes, focussing on the combinations in cooperative catalytic systems in strategies used to achieve selectivity and also highlights the mode of action for the cooperative catalysis leading to the synthesis of commercially and biologically relevant heteroarenes.
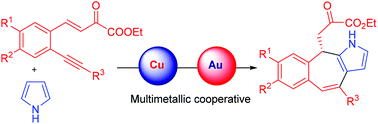
Org. Biomol. Chem., 2019,17, 7596-7631
https://doi.org/10.1039/C9OB01152H
Combining enzymes and organometallic complexes: novel artificial metalloenzymes and hybrid systems for C–H activation chemistry
This review describes the advances in the design and application of novel artificial metalloenzymes in C–H activation reactions.
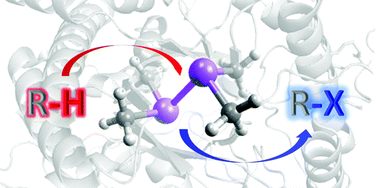
Org. Biomol. Chem., 2019,17, 7114-7123
https://doi.org/10.1039/C9OB01091B
Reversal of enantioselectivity in chiral metal complex-catalyzed asymmetric reactions
The achievements of chiral metal complex-catalyzed enantiodivergent reactions during the period from 2008 to present were updated.
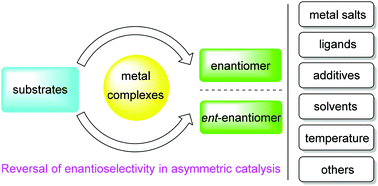
Org. Biomol. Chem., 2019,17, 6538-6550
https://doi.org/10.1039/C9OB01027K
Introduction to chemical warfare agents, relevant simulants and modern neutralisation methods
This short review presents the current main chemical warfare agents and their most relevant simulants, and the recent catalytic and selective methods for their soft neutralization, potentially usable in the future as an alternative to “heavy” methods for decontamination.
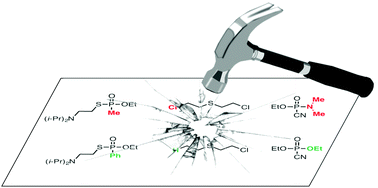
Org. Biomol. Chem., 2019,17, 6528-6537
https://doi.org/10.1039/C9OB00802K
Chiral palladium pincer complexes for asymmetric catalytic reactions
This review covers the methods for the preparation of chiral palladium(II) pincer complexes and their applications in asymmetric catalysis.
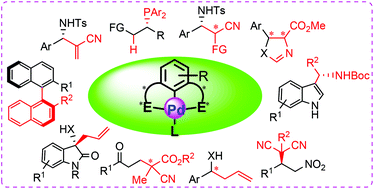
Org. Biomol. Chem., 2019,17, 6069-6098
https://doi.org/10.1039/C9OB00401G
Metal-catalysed reactions enabled by guanidine-type ligands
A review of metal–guanidine complexes, which are selective and powerful catalysts for organic transformations, asymmetric synthesis, and polymerisation.
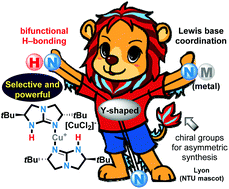
Org. Biomol. Chem., 2019,17, 4689-4699
https://doi.org/10.1039/C8OB02240B
Recent advances in photocatalytic manipulations of Rose Bengal in organic synthesis
This review highlights the recent advances in photocatalytic manipulations of Rose Bengal in organic synthesis.
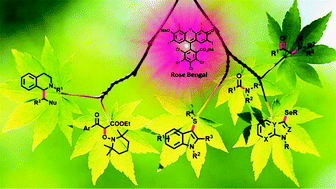
Org. Biomol. Chem., 2019,17, 4384-4405
https://doi.org/10.1039/C9OB00092E
Catalytic asymmetric cycloaddition reactions of enoldiazo compounds
Review of recent advances in asymmetric catalytic cycloaddition reactions of silyl-protected enoldiazo compounds.
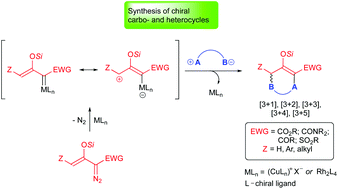
Org. Biomol. Chem., 2019,17, 4183-4195
https://doi.org/10.1039/C9OB00478E
The mechanistic duality of (thio)urea organocatalysts for ring-opening polymerization
Dual mechanisms for H-bond mediated ring-opening polymerization allow for precise control, high activity and new applications.
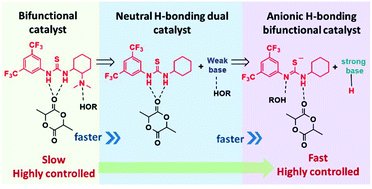
Org. Biomol. Chem., 2019,17, 3305-3313
https://doi.org/10.1039/C8OB03174F
The roles of Lewis acidic additives in organotransition metal catalysis
We present recent advances in prominent organotransition metal-catalysed reactions in which Lewis acid cocatalysts are employed to increase catalyst activity or selectivity. The roles of Lewis acids are discussed.
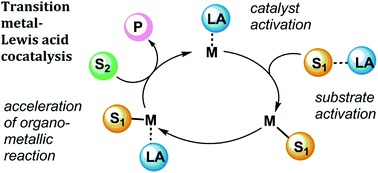
Org. Biomol. Chem., 2019,17, 2055-2069
https://doi.org/10.1039/C8OB02856G
N-Alkyl amide synthesis via N-alkylation of amides with alcohols
The present review summarizes the recent development of N-alkylation of amides with alcohols according to the classification of catalysts.

Org. Biomol. Chem., 2019,17, 2044-2054
https://doi.org/10.1039/C8OB03091J
Beyond the traditional roles of Ag in catalysis: the transmetalating ability of organosilver(I) species in Pd-catalysed reactions
The role of silver salts in Pd-catalysed C–C bond forming transformations.
Org. Biomol. Chem., 2019,17, 1655-1667
https://doi.org/10.1039/C8OB02611D
The challenge of using isopropylamine as an amine donor in transaminase catalysed reactions
Factors that affect the efficiency of amine transaminase catalyzed reactions using isopropylamine as an amine donor.
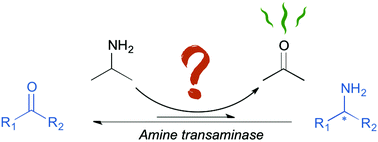
Org. Biomol. Chem., 2019,17, 1634-1642
https://doi.org/10.1039/C8OB02342E
Recent advances in transition metal-catalyzed C(sp2)–H nitration
This review updates advances of direct C(sp2)–H nitration for the synthesis of nitroaromatic compounds and the mechanisms during the past decade.
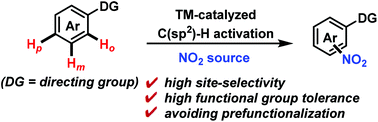
Org. Biomol. Chem., 2019,17, 1351-1361
https://doi.org/10.1039/C8OB02750A
Recent developments in photochemical reactions of diazo compounds
Chemistry of diazo compounds is dominated by transition metal catalysis but recently, photoinitiated reactions of diazo compounds have attracted a lot of attention. This mini-review describes recent discoveries on the reactivity of diazo compounds under light irradiation.
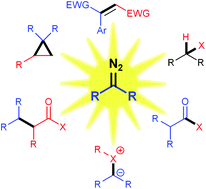
Org. Biomol. Chem., 2019,17, 432-448
https://doi.org/10.1039/C8OB02703J
The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art
This Review article provides a summary and critical metrics-based analysis of recently developed catalytic Mitsunobu reactions.
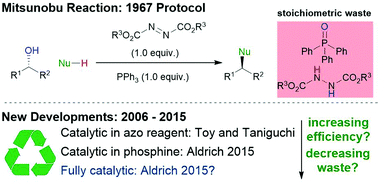
Org. Biomol. Chem., 2018,16, 7774-7781
https://doi.org/10.1039/C8OB01929K
Indium(III) as π-acid catalyst for the electrophilic activation of carbon–carbon unsaturated systems
This review focuses on indium(III) as a π-acid for the activation of C–C unsaturated systems (alkynes, alkenes, and allenes) in organic synthesis.
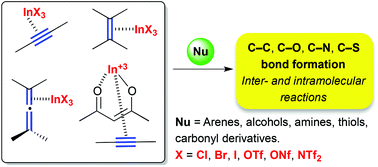
Org. Biomol. Chem., 2018,16, 5733-5747
https://doi.org/10.1039/C8OB01426D
Dibenzofuran-4,6-bis(oxazoline) (DBFOX). A novel trans-chelating bis(oxazoline) ligand for asymmetric reactions
The trans-chelating bis(oxazoline) ligand (R,R)-4,6-dibenzofurandiyl-2,2′-bis(4-phenyloxazoline) [(R,R)-DBFOX/Ph] coordinates metal ions to give C2-symmetric complexes which effectively catalyze a variety of asymmetric reactions.
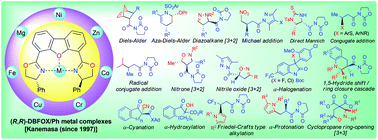
Org. Biomol. Chem., 2018,16, 5551-5565
https://doi.org/10.1039/C8OB01010B
Asymmetric iodine catalysis-mediated enantioselective oxidative transformations
The implementation of chiral iodine catalysis has tremendously been developed in the field of asymmetric synthesis over the past decade.
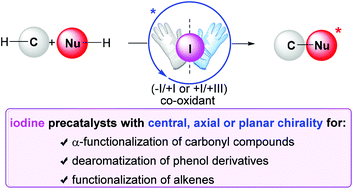
Org. Biomol. Chem., 2018,16, 5386-5402
https://doi.org/10.1039/C8OB01378K
Development and application of chiral spirocyclic phosphoric acids in asymmetric catalysis
This review describes the synthetic methods for the preparation of chiral spirocyclic phosphoric acids (SPAs), and their dynamically developing application for catalytic enantioselective transformations.
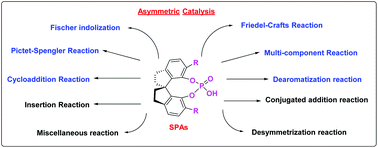
Org. Biomol. Chem., 2018,16, 4753-4777
https://doi.org/10.1039/C8OB00900G
Halo-substituted benzenesulfonyls and benzenesulfinates: convenient sources of arenes in metal-catalyzed C–C bond formation reactions for the straightforward access to halo-substituted arenes
The use of halo-substituted ArSO2R as an aryl source in metal-catalyzed C–C bond formation reactions presents several advantages, as the reaction often proceeds without cleavage of the C–halo bonds.
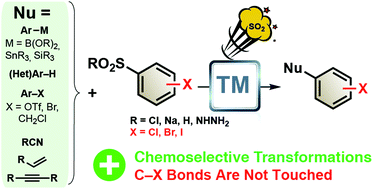
Org. Biomol. Chem., 2018,16, 4399-4423
https://doi.org/10.1039/C8OB00632F
Chiral proton-transfer shuttle catalysts for carbene insertion reactions
The development of chiral proton-transfer shuttles provides a totally new enantiocontrol strategy for transition metal-catalyzed asymmetric carbene insertion reactions.
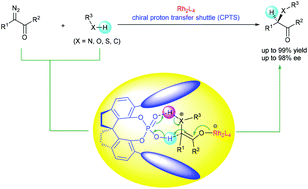
Org. Biomol. Chem., 2018,16, 3087-3094
https://doi.org/10.1039/C8OB00473K
Recent advances in iodine mediated electrochemical oxidative cross-coupling
This review article gives an overview of the recent development of iodine mediated electrochemical oxidative coupling reactions.
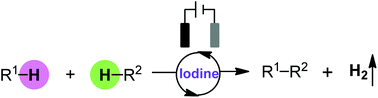
Org. Biomol. Chem., 2018,16, 2375-2387
https://doi.org/10.1039/C8OB00063H
Catalytic asymmetric enamine protonation reaction
Recent advances in catalytic enantioselective enamine protonation for the synthesis of optically active carbonyl compounds are summarized in this review.
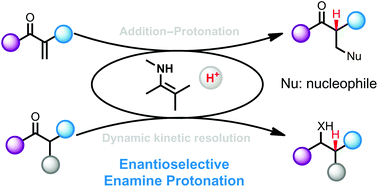
Org. Biomol. Chem., 2018,16, 510-520
https://doi.org/10.1039/C7OB02615C
Enhanced structural diversity in terpenoid biosynthesis: enzymes, substrates and cofactors
Terpenoid structural diversity is enhanced by multiproduct enzymes. Biosynthesis can be altered by switch in substrates, cofactors and pH.
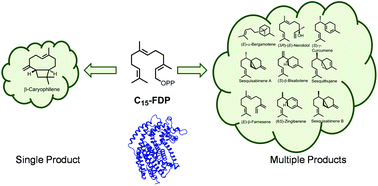
Org. Biomol. Chem., 2018,16, 348-362
https://doi.org/10.1039/C7OB02040F
About this collection
The latest articles published in Organic and Biomolecular Chemistry related to catalysis and biocatalysis research.