Deoxygenation reactions in organic synthesis catalyzed by dioxomolybdenum(vi) complexes
Abstract
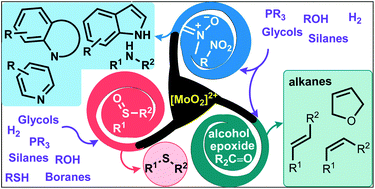
Dioxomolybdenum(VI) complexes have been applied as efficient, inexpensive and benign catalysts to deoxygenation reactions of a diverse number of compounds in the last two decades. Dioxomolybdenum complexes have demonstrated wide applicability to the deoxygenation of sulfoxides into sulfides and reduction of N–O bonds. Even the challenging nitro functional group was efficiently deoxygenated, affording amines or diverse heterocycles after reductive cyclization reactions. More recently, carbon-based substrates like epoxides, alcohols and ketones have been successfully deoxygenated. Also, dioxomolybdenum complexes accomplished deoxydehydration (DODH) reactions of biomass-derived vicinal 1,2-diols, affording valuable alkenes. The choice of the catalytic systems and reductant is decisive to achieve the desired transformation. Commonly found reducing agents involved phosphorous-based compounds, silanes, molecular hydrogen, or even glycols and other alcohols.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...