Copper-catalysed radical reactions of alkenes, alkynes and cyclopropanes with N–F reagents
Abstract
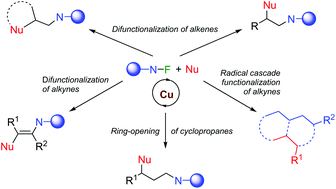
The mild generation of nitrogen-centred radicals from N–F reagents has become a convenient synthetic tool. This methodology provides access to the aminative difunctionalisation of alkenes and alkynes and the radical ring-opening of cyclopropanes, among other similar transformations. This review article aims to provide an overview of recent developments of such processes involving radical reactions and N–F reagents using copper-based catalysts.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...