Biocatalytic approaches for enantio and diastereoselective synthesis of chiral β-nitroalcohols†
Abstract
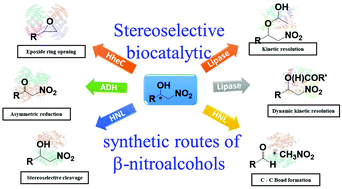
Chiral β-nitroalcohols find significant application in organic synthesis due to the versatile reactivity of hydroxyl and nitro functionalities attached to one or two vicinal asymmetric centers. They are key building blocks of several important pharmaceuticals, bioactive molecules, and fine chemicals. With the growing demand to develop clean and green methods for their synthesis, biocatalytic methods have gained tremendous importance among the existing asymmetric synthesis routes. Over the years, different biocatalytic strategies for the asymmetric synthesis of β-nitroalcohol stereoisomers have been developed. They can be majorly classified as (a) kinetic resolution, (b) dynamic kinetic resolution, (c) Henry reaction, (d) retro-Henry reaction, (e) asymmetric reduction, and (f) enantioselective epoxide ring-opening. This review aims to provide an overview of the above biocatalytic strategies, and their comparison along with future prospects. Essentially, it presents an enzyme-toolbox for the asymmetric synthesis of β-nitroalcohol enantiomers and diastereomers.
- This article is part of the themed collections: Biocatalysis: A cross-journal collection and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...