Enhanced structural diversity in terpenoid biosynthesis: enzymes, substrates and cofactors
Abstract
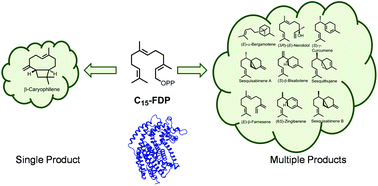
The enormous diversity of terpenes found in nature is generated by enzymes known as terpene synthases, or cyclases. Some are also known for their ability to convert a single substrate into multiple products. This review comprises monoterpene and sesquiterpene synthases that are multiproduct in nature along with the regulation factors that can alter the product specificity of multiproduct terpene synthases without genetic mutations. Variations in specific assay conditions with focus on shifts in product specificity based on change in metal cofactors, assay pH and substrate geometry are described. Alterations in these simple cellular conditions provide the organism with enhanced chemodiversity without investing into new enzymatic architecture. This versatility to modulate product diversity grants organisms, especially immobile ones like plants with access to an enhanced defensive repertoire by simply altering cofactors, pH level and substrate geometry.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...