Chiral disulfonimides: a versatile template for asymmetric catalysis
Abstract
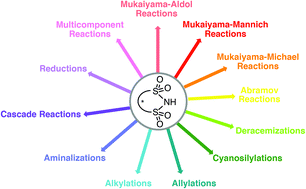
Since the emergence of pseudo-C2-symmetric chiral phosphoric acids (CPA), much work has been done to utilize these systems in stereoselective, organocatalytic processes. Despite the success in this field, reasonably basic substrates such as imines are often required to achieve appreciable activation. In order to access a wider variety of potential reaction partners, many related organocatalysts with enhanced Brønsted acidity have since been developed. Chiral disulfonimides (DSIs) have materialized as one such powerful class of organocatalysts and have been shown to expand the list of potential substrates to include aldehydes and ketones via Brønsted, Lewis, or bifunctional acid activation. This versatility renders DSIs amenable to an impressive scope of reaction types, typically with remarkable stereoselectivity induced by asymmetric counteranion-directed catalysis (ACDC). This review serves to provide a complete analysis of the successful applications, mechanistic insights, and unmet challenges exhibited to date in DSI-catalyzed and -assisted processes.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...