The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art
Abstract
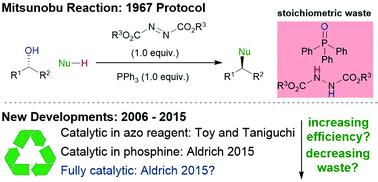
The Mitsunobu reaction is widely regarded as the pre-eminent method for performing nucleophilic substitutions of alcohols with inversion of configuration. However, its applicability to large-scale synthesis is undermined by the fact that alcohol activation occurs at the expense of two stoichiometric reagents – a phosphine and an azodicarboxylate. The ideal Mitsunobu reaction would be sub-stoichiometric in the phosphine and azodicarboxylate species and employ innocuous terminal oxidants and reductants to achieve recycling. This Review article provides a summary and analysis of recent advances towards the development of such catalytic Mitsunobu reactions.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...