The roles of Lewis acidic additives in organotransition metal catalysis
Abstract
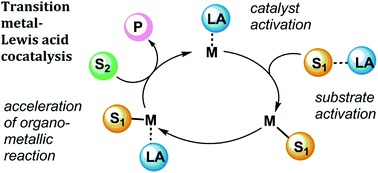
We describe recent examples of prominent reactions in organic synthesis that involve transition metal and Lewis acid cocatalysts. Introducing Lewis acid additives to transition metal catalysis can enable new reactivity or improve activity and selectivity of an existing process. Several studies are highlighted to illustrate the possible roles of Lewis acids in catalytic mechanisms. The uses of Lewis acid additives in bond-breaking catalysis (C–C activation, C–H activation, and hydrogenolysis reactions) and bond-forming catalysis (Au-catalysed alkyne functionalisation, Pd-catalysed C–C and C–N bond formation) are reviewed.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...