Themed collection Organic Chemistry Frontiers HOT articles for 2018

Direct conjugate additions using aryl and alkyl organic halides in air and water
The direct aryl-conjugate addition to electron-deficient alkenes without prior stoichiometric formation of organometallic reagents in water catalyzed by copper represents an important but unresolved challenge.
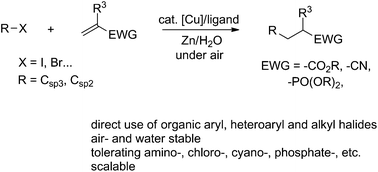
Org. Chem. Front., 2018,5, 3579-3584
https://doi.org/10.1039/C8QO01141A
Ni-Catalyzed 1,2-iminoacylation of alkenes via a reductive strategy
The reductive difunctionalization strategy was successfully applied in the Ni-catalyzed 1,2-iminoacylation reaction of oxime ester-tethered olefins with electrophilic acylating reagents, providing an efficient entry to diverse pyrrolines under safe and mild reaction conditions.
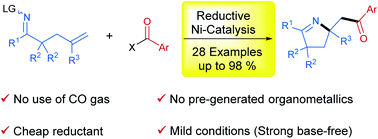
Org. Chem. Front., 2018,5, 3476-3482
https://doi.org/10.1039/C8QO01044G
Direct construction of benzimidazo[l,2-c]quinazolin-6-ones via metal-free oxidative C–C bond cleavage
A highly regioselective protocol has been developed for the synthesis of benzimidazo[l,2-c]quinazolin-6-ones via C–C bond cleavage and triple C–N bond formation.
![Graphical abstract: Direct construction of benzimidazo[l,2-c]quinazolin-6-ones via metal-free oxidative C–C bond cleavage](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO01039K&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 3464-3468
https://doi.org/10.1039/C8QO01039K
Dual roles of ynoates: desymmetrization of dicarboxylic acids using trialkylamines as alkyl equivalents
A novel method has been developed for the desymmetrization of aromatic dicarboxylic acids by employing an esterification reaction/conjugate addition of a carboxyl group to ynoates, which can trigger a coupling reaction.

Org. Chem. Front., 2018,5, 2955-2959
https://doi.org/10.1039/C8QO00919H
Iridium-catalyzed oxidative Ar–H/Ar–H cross-coupling of primary benzamides with thiophenes
Described herein is an iridium(III)-catalyzed oxidative Ar–H/Ar–H cross-coupling of primary benzamides with thiophenes for the synthesis of (2-thienyl)benzamides.
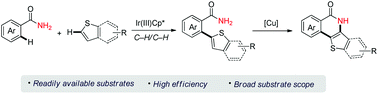
Org. Chem. Front., 2018,5, 2930-2933
https://doi.org/10.1039/C8QO00796A
Enantioselective isoquinuclidine synthesis via sequential Diels–Alder/visible-light photoredox C–C bond cleavage: a formal synthesis of the indole alkaloid catharanthine
An enantioselective formation of isoquinuclidines useful for alkaloid synthesis is achieved through an organocatalyzed Diels–Alder reaction of dihydropyridines with acrolein and a subsequent photoredox catalyzed oxidative deformylation reaction.

Org. Chem. Front., 2018,5, 2934-2939
https://doi.org/10.1039/C8QO00849C
A triple-functionalised metal centre-catalyzed enantioselective multicomponent reaction
A model of a triple-functionalised metal centre for the discovery of unprecedented enantioselective reactions was exquisitely developed by using a RhI/(DHQ)2PHAL catalyst system via multiple coordination interactions with different ligands and substrates.

Org. Chem. Front., 2018,5, 2799-2804
https://doi.org/10.1039/C8QO00703A
Insights into N-heterocyclic carbene-catalyzed [3 + 4] annulation reactions of 2-bromoenals with N-Ts hydrazones
A DFT study toward N-heterocyclic carbene-catalyzed [3 + 4] annulation reactions of 2-bromoenals has been performed for the first time.
![Graphical abstract: Insights into N-heterocyclic carbene-catalyzed [3 + 4] annulation reactions of 2-bromoenals with N-Ts hydrazones](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00716K&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 2739-2748
https://doi.org/10.1039/C8QO00716K
Photoinduced fragmentation-rearrangement sequence of cycloketoxime esters
A novel rearrangement of cycloketoxime esters to cyano-containing benzoates beyond the traditional Beckmann and Neber rearrangement process has been established in the presence of photocatalysis catalysis.
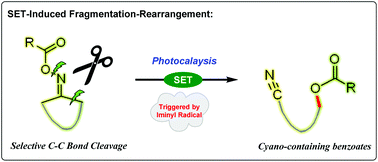
Org. Chem. Front., 2018,5, 2719-2722
https://doi.org/10.1039/C8QO00747K
The mechanism of the excited-state multiple proton transfer reaction for 3-Me-2,6-diazaindole in aqueous solution
The potential energy curves show that (2,6-aza)Ind in aqueous solution undergoes a quadruple-proton transfer reaction with the assistance of three water molecules.

Org. Chem. Front., 2018,5, 2749-2753
https://doi.org/10.1039/C8QO00628H
Asymmetric Diels–Alder cycloadditions of benzofulvene-based 2,4-dienals via trienamine activation
A new type of 2,4-dienal featuring a benzofulvene skeleton is utilised in asymmetric Diels–Alder cycloadditions with 3-olefinic oxindoles via trienamine catalysis.
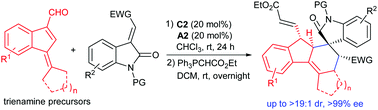
Org. Chem. Front., 2018,5, 2676-2679
https://doi.org/10.1039/C8QO00653A
Rh-Catalyzed regioselective C–H activation and C–C bond formation: synthesis and photophysical studies of indazolo[2,3-a]quinolines
Rh(III)-Catalyzed oxidative annulation of 2-aryl-2H-indazoles with alkynes and their photophysical studies are reported with high quantum yields.
![Graphical abstract: Rh-Catalyzed regioselective C–H activation and C–C bond formation: synthesis and photophysical studies of indazolo[2,3-a]quinolines](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00557E&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 2630-2635
https://doi.org/10.1039/C8QO00557E
Visible-light photocatalytic trifluoromethylthiolation of aryldiazonium salts: conversion of amino group into trifluoromethylthiol group
A visible-light photocatalytic trifluoromethylthiolation of aryl amine through in situ generation of aryldiazonium salts with S-trifluoromethyl 4-methoxylbenzenesulfonothioate was developed.

Org. Chem. Front., 2018,5, 2636-2640
https://doi.org/10.1039/C8QO00401C
Bromide-catalyzed electrochemical trifluoromethylation/cyclization of N-arylacrylamides with low catalyst loading
An electro-generated trifluoromethyl radical mediated by a bromide ion is efficiently applied to the trifluoromethylarylation/cyclization of N-arylacrylamides.
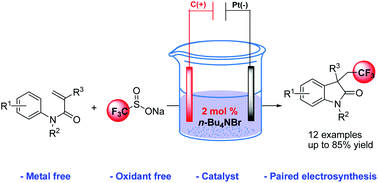
Org. Chem. Front., 2018,5, 2573-2577
https://doi.org/10.1039/C8QO00645H
E-Selective N-heterocyclic carbene-catalyzed reaction of aldehydes and butadienoates: effect of water and chloroform as the proton shuttle
An N-heterocyclic carbene-catalyzed reaction of aldehydes and butadienoates affording (E)-4-oxo-2-butenoates highly stereoselectively has been developed. Some key intermediates have been detected by an MS study.

Org. Chem. Front., 2018,5, 2560-2567
https://doi.org/10.1039/C8QO00579F
Convergent syntheses of 2,3-dihydrobenzofurans via a Catellani strategy
The annulation of aryl iodides and epoxides to convergently access valuable 2,3-dihydrobenzofuran scaffolds was developed via a Catellani strategy.
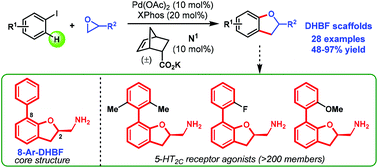
Org. Chem. Front., 2018,5, 2533-2536
https://doi.org/10.1039/C8QO00348C
Cu(I)-Catalyzed stereoselective synthesis of trisubstituted Z-enol esters via interrupting the 1,3-O-transposition reaction
An efficient Cu(I)-catalyzed stereoselective synthesis of trisubstituted Z-enol esters via interrupting the 1,3-O-transposition process is reported, which provided a convenient approach to highly functionalized Z-enol esters.
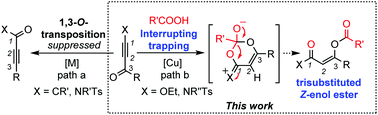
Org. Chem. Front., 2018,5, 2510-2514
https://doi.org/10.1039/C8QO00664D
Unsymmetrical CNN-palladacycles with geometry-constrained iminopyridyl ligands: an efficient precatalyst in Suzuki coupling for accessing 1,1-diarylalkanes from secondary benzylic bromides
We developed a series of unsymmetrical CNN palladacycles with geometry-constrained iminopyridyl ligands, which were used as efficient precatalysts for preparing diarylalkanes through Suzuki coupling.
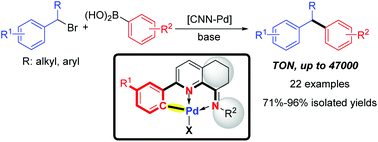
Org. Chem. Front., 2018,5, 2484-2491
https://doi.org/10.1039/C8QO00517F
Pd(II)-Catalyzed [3 + 2] spiroannulation of α-aryl-β-naphthols with alkynes via a C–H activation/dearomatization approach
A Pd(II)-catalyzed [3 + 2] spiroannulation of α-aryl-β-naphthols with internal alkynes has been developed by relying on a C–H activation/arene dearomatization approach.
![Graphical abstract: Pd(ii)-Catalyzed [3 + 2] spiroannulation of α-aryl-β-naphthols with alkynes via a C–H activation/dearomatization approach](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00614H&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 2453-2457
https://doi.org/10.1039/C8QO00614H
Highly enantioselective desymmetrization of prochiral cyclic α,α-dicyanoalkenes via the direct vinylogous Michael/cyclization domino reaction
A chiral N,N′-dioxide/Mg(II) complex efficiently constructs spiroindolinones which include three adjacent atom chiralities and a tertiary carbon center at remote sites in up to 95% yield, with 99% ee and 20 : 1 dr.
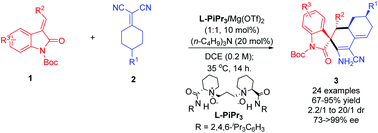
Org. Chem. Front., 2018,5, 2505-2509
https://doi.org/10.1039/C8QO00545A
A facile synthesis of diverse 5-arylated triazoles via a Cu-catalyzed oxidative interrupted click reaction with arylboronic acids in air
A Cu-catalyzed synthesis of 5-arylsubstituted 1,2,3-triazoles via an oxidative interrupted click reaction with arylboronic acids in air at room temperature is disclosed.
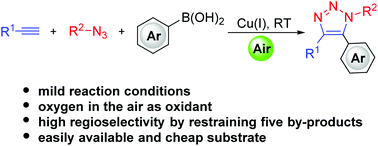
Org. Chem. Front., 2018,5, 2463-2467
https://doi.org/10.1039/C8QO00590G
Asperones A–E, five dimeric polyketides with new carbon skeletons from the fungus Aspergillus sp. AWG 1–15
Five dimeric polyketides with two novel skeletons, generated by the crucial [3 + 2] and [3 + 3] cycloadditions, were isolated from Aspergillus sp.
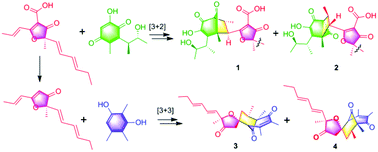
Org. Chem. Front., 2018,5, 2432-2436
https://doi.org/10.1039/C8QO00070K
Structurally diverse diterpenoids from Isodon pharicus
Twenty-one structurally diverse diterpenoids (1–21), wherein 1, 2, and 4 represented unprecedented architectures, were isolated from Isodon pharicus.
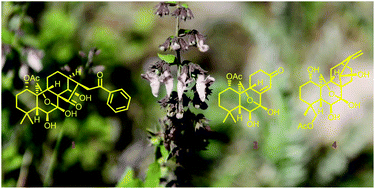
Org. Chem. Front., 2018,5, 2379-2389
https://doi.org/10.1039/C8QO00477C
Green carbon disulfide surrogate via a combination of potassium sulfide and chloroform for benzothiazine-thione and benzothiazole-thione construction
An efficient and green carbon disulfide surrogate via facile combination of potassium sulfide and chloroform has been developed.
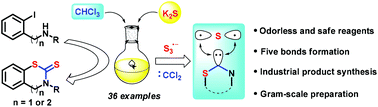
Org. Chem. Front., 2018,5, 2390-2394
https://doi.org/10.1039/C8QO00481A
Phosphinoyl-functionalization of unactivated alkenes through phosphinoyl radical-triggered distal functional group migration
Reported herein is a novel, mild, and practical protocol for the radical-mediated phosphinoyl-functionalization of unactivated alkenes through distal functional group migration.
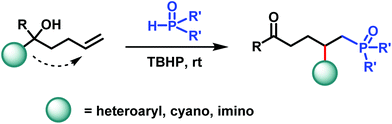
Org. Chem. Front., 2018,5, 2370-2374
https://doi.org/10.1039/C8QO00558C
Catalytic enantioselective α-sulfenylation of β-ketocarbonyls by chiral primary amines
A simple chiral primary amine catalyst was identified to enable effective catalysis in direct α-sulfenylation of acyclic and cyclic β-ketocarbonyls with good yields and excellent enantioselectivities.

Org. Chem. Front., 2018,5, 2313-2316
https://doi.org/10.1039/C8QO00496J
Photoexcited perylene diimide radical anions for the reduction of aryl halides: a bay-substituent effect
Photoexcited perylene diimide radical anions exhibit remarkable substituent-dependent photocatalytic activities towards the reduction of aryl halides, which are mainly controlled by their excited-state reduction potentials and SOMO−1 energies.
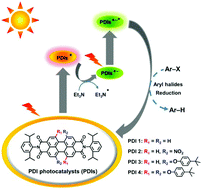
Org. Chem. Front., 2018,5, 2296-2302
https://doi.org/10.1039/C8QO00445E
Palladium-catalyzed reductive electrocarboxylation of allyl esters with carbon dioxide
Palladium-catalyzed regioselective electrocarboxylation of homostyrenyl acetates with CO2 has been successfully developed, providing α-aryl carboxylic acids with good selectivity and yield.
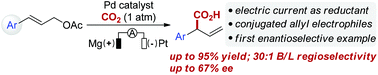
Org. Chem. Front., 2018,5, 2244-2248
https://doi.org/10.1039/C8QO00507A
Access to sulfonylated furans or imidazo[1,2-a]pyridines via a metal-free three-component, domino reaction
An efficient three-component reaction for the synthesis of sulfonylated furans or imidazo[1,2-a]pyridines has been developed.
![Graphical abstract: Access to sulfonylated furans or imidazo[1,2-a]pyridines via a metal-free three-component, domino reaction](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00443A&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 2219-2223
https://doi.org/10.1039/C8QO00443A
Dysohonin A, a meroditerpenoid incorporating a 6,15,6-fused heterotricyclic ring system from Dysoxylum hongkongense
Dysohonin A (1), a meroditerpenoid incorporating an unprecedented architecture, along with three new biogenetically related meroditerpenoids as PTP1B inhibitors were reported in this paper.

Org. Chem. Front., 2018,5, 2202-2207
https://doi.org/10.1039/C8QO00469B
Chemical synthesis and biological evaluation of penta- to octa- saccharide fragments of Vi polysaccharide from Salmonella typhi
The penta- to octa-saccharide fragments of Vi polysaccharide were synthesized efficiently, and the hexasaccharide might be the minimum epitope of Vi antigen based on ELISA analysis.
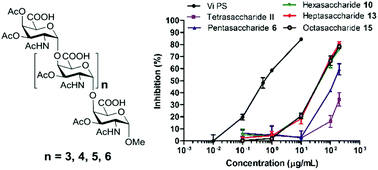
Org. Chem. Front., 2018,5, 2179-2188
https://doi.org/10.1039/C8QO00471D
Facile synthesis of macrocyclic peptide toxins of GpTx-1 and its analogue
GpTx-1 and its analogue GpTx-71-1 were synthesized by a flexible and highly practical strategy via converging three segments based on C-terminal proline residues.
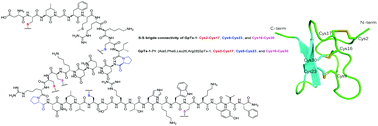
Org. Chem. Front., 2018,5, 2143-2147
https://doi.org/10.1039/C8QO00415C
Diastereoselectivity in a cyclic secondary amine catalyzed asymmetric Mannich reaction: a model rationalization from DFT studies
DFT studies revealed the detailed structure stereoselectivity relationship for cyclic secondary amine catalyzed asymmetric Mannich reactions.
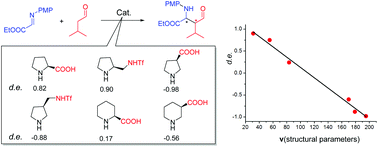
Org. Chem. Front., 2018,5, 2148-2157
https://doi.org/10.1039/C8QO00424B
Asymmetric [4 + 2] cycloadditions with 3-furfural derivatives and α-cyano-α,β-unsaturated ketones
An asymmetric [4 + 2] cycloaddition reaction with 2-benzyl-3-furfurals and α-cyano-chalcones was developed to afford chiral tetrahydrobenzofurans having dense substitutions.
![Graphical abstract: Asymmetric [4 + 2] cycloadditions with 3-furfural derivatives and α-cyano-α,β-unsaturated ketones](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00351C&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 2057-2060
https://doi.org/10.1039/C8QO00351C
The iridium-catalysed reductive coupling reaction of tertiary lactams/amides with isocyanoacetates
A catalytic reductive addition of isocyanoacetates to tertiary lactams/amides has been developed. This one-pot procedure involves Ir-catalysed partial reduction of lactams/amides and sequential chemoselective addition of isocyanide group in isocyanoacetates and produces 5-methoxyoxazoles in moderate to excellent yields.
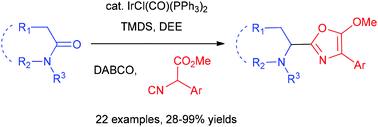
Org. Chem. Front., 2018,5, 2051-2056
https://doi.org/10.1039/C8QO00312B
Three-component reaction to synthesize E-vinyl silyl anti-1,2-diols via sequential [1,4]-O-to-O/[1,4]-C-to-O silyl migrations
A three-component reaction of geminal bis(silyl) allyl silyl ether, aldehyde and electrophile has been developed to synthesize trisubstituted E-vinyl silyl anti-1,2-diols. The approach features a sequential [1,4]-O-to-O/[1,4]-C-to-O silyl migrations process.
![Graphical abstract: Three-component reaction to synthesize E-vinyl silyl anti-1,2-diols via sequential [1,4]-O-to-O/[1,4]-C-to-O silyl migrations](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00332G&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 2035-2039
https://doi.org/10.1039/C8QO00332G
A more reliable synthesis of a Gemini vitamin D analog, a potentially effective chemotherapeutic agent for the treatment of colorectal carcinomas
After a serendipitous synthesis of a potentially effective chemotherapeutic agent for the treatment of colorectal carcinomas, a more reliable synthetic method is now described using our building blocks containing stereodefined stereochemistry at C-20.
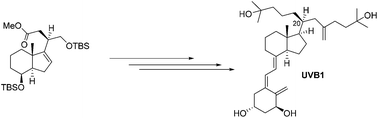
Org. Chem. Front., 2018,5, 2016-2019
https://doi.org/10.1039/C8QO00338F
Selenium dioxide-promoted selective synthesis of mono- and bis-sulfenylindoles
Easy access to mono- and bis-sulfenylindoles using the SeO2/I2 system.
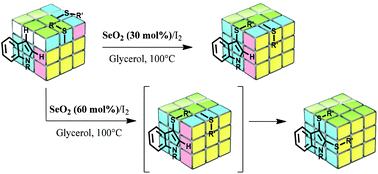
Org. Chem. Front., 2018,5, 1983-1991
https://doi.org/10.1039/C8QO00360B
Highly enantioselective Ir/f-amphox-catalyzed hydrogenation of ketoamides: efficient access to chiral hydroxy amides
Asymmetric synthesis of chiral hydroxy amides has been successfully accomplished by asymmetric hydrogenation of prochiral α-, β-, γ-, δ-keto amides catalyzed by Ir/f-amphox with up to >99% conversion and >99% ee.
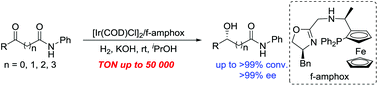
Org. Chem. Front., 2018,5, 2000-2003
https://doi.org/10.1039/C8QO00307F
Selective formation of phthalimides from amines, aldehydes and CO by Pd-catalyzed oxidative C–H aminocarbonylation
The Pd/Cu-catalyzed intramolecular C–H bond aminocarbonylation utilizing imines as the amine source has been developed. This transformation provides an efficient and straightforward protocol for the synthesis of phthalimides which widely exist in natural products, pharmaceutical and functional materials. Various functional groups are tolerated and the yields are up to 99%.
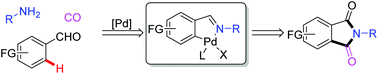
Org. Chem. Front., 2018,5, 1957-1961
https://doi.org/10.1039/C8QO00282G
Azidoheteroarylation of unactivated olefins through distal heteroaryl migration
A novel azidoheteroarylation of unactivated olefins by means of intramolecular distal heteroaryl migration is herein reported.
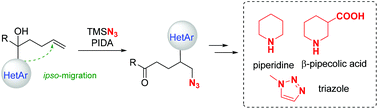
Org. Chem. Front., 2018,5, 1896-1899
https://doi.org/10.1039/C8QO00301G
The design of rigid cyclic tripyrrins: the importance of intermolecular interactions on aggregation and luminescence
Cyclic tripyrrin “locked” by a bridging benzyl moiety: enhancing the molecular rigidity and tuning aggregation and fluorescence via intermolecular halogen interactions.
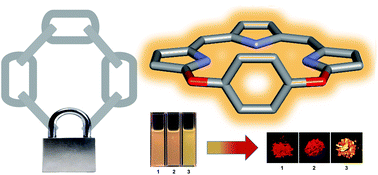
Org. Chem. Front., 2018,5, 1877-1885
https://doi.org/10.1039/C8QO00313K
Efficient catalytic vicinal diamination of arylene diimides
New substitution patterns for rylene diimides by catalytic diamination.
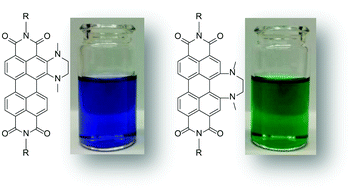
Org. Chem. Front., 2018,5, 1825-1829
https://doi.org/10.1039/C8QO00222C
Iridium-catalysed conjugated alkynylation of α,β-unsaturated amide through alkene isomerization
We have developed an iridium-catalysed conjugated alkynylation of α,β-unsaturated amide.
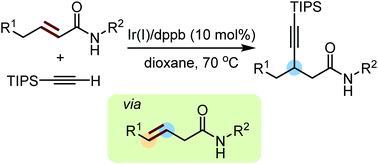
Org. Chem. Front., 2018,5, 1815-1819
https://doi.org/10.1039/C8QO00136G
Enantioselective indium(I)-catalyzed [4 + 2] annulation of alkoxyallenes and β,γ-unsaturated α-keto esters
An indium(I)–chiral phosphoric acid complex was found to catalyze the enantioselective [4 + 2] annulation reaction of β,γ-unsaturated α-keto esters with alkoxyallenes, affording cyclic O,O-acetals in good yields and with high regio- and stereo-selectivities.
![Graphical abstract: Enantioselective indium(i)-catalyzed [4 + 2] annulation of alkoxyallenes and β,γ-unsaturated α-keto esters](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00319J&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 1787-1791
https://doi.org/10.1039/C8QO00319J
Asymmetric total syntheses of callistrilones B, G and J
A highly concise approach for the first asymmetric and gram-scale total syntheses of callistrilones B, G and J is reported.
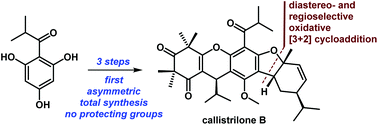
Org. Chem. Front., 2018,5, 1506-1510
https://doi.org/10.1039/C8QO00130H
Photoredox-catalyzed cascade annulation of methyl(2-(phenylethynyl)phenyl)sulfanes and methyl(2-(phenylethynyl)phenyl)selanes with sulfonyl chlorides: synthesis of benzothiophenes and benzoselenophenes
A photoredox-catalyzed cascade annulation of methyl(2-(phenylethynyl)phenyl)sulfanes and methyl(2-(phenylethynyl)phenyl)selanes with sulfonyl chlorides was developed.
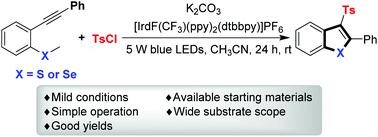
Org. Chem. Front., 2018,5, 1483-1487
https://doi.org/10.1039/C8QO00147B
Catalyst-controlled synthesis of 4-amino-isoquinolin-1(2H)-one and oxazole derivatives
A facile synthetic method for the preparation of oxazole and 4-amino-isoquinolin-1(2H)-one derivatives has been developed from the reaction of N-(pivaloyloxy)-amides and ynamides by using different metal catalysts.
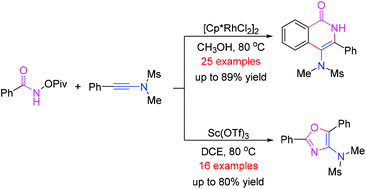
Org. Chem. Front., 2018,5, 1466-1470
https://doi.org/10.1039/C8QO00125A
Silver-catalyzed ring-opening difluoromethylthiolation/trifluoromethylthiolation of cycloalkanols with PhSO2SCF2H or PhSO2SCF3
A silver-catalyzed ring-opening difluoromethylthiolation/trifluoromethylthiolation of cycloalkanols including cyclopropanols, cyclobutanols, cyclopentanols, cyclohexanols and cycloheptanols was described.

Org. Chem. Front., 2018,5, 1462-1465
https://doi.org/10.1039/C8QO00115D
Mechanistic insights into the different chemoselectivities of Rh2(II)-catalyzed ring expansion of cyclobutanol-substituted aryl azides and C–H bond amination of cyclopentanol-substituted aryl azides: a DFT study
The mechanism of Rh2(II)-catalyzed ring expansion of ortho-cyclobutanol-substituted aryl azides to access N-heterocycles was investigated by DFT calculations.
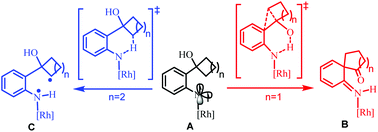
Org. Chem. Front., 2018,5, 1471-1482
https://doi.org/10.1039/C8QO00113H
Total synthesis of teixobactin and its stereoisomers
The total syntheses of teixobactin and a series of its stereoisomers at positions 2, 5, 6, 10 and 11 were achieved via a combined strategy of solution and solid phase peptide synthesis.
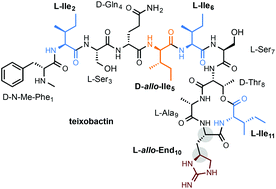
Org. Chem. Front., 2018,5, 1431-1435
https://doi.org/10.1039/C8QO00145F
Efficient access to chiral benzo[c]chromenes via asymmetric transfer hydrogenation of ketals
A highly enantioselective method to chiral α-substituted 6H-benzo[c]chromenes through chiral imidodiphosphoric acid-catalyzed asymmetric transfer hydrogenation of ketals has been established.
![Graphical abstract: Efficient access to chiral benzo[c]chromenes via asymmetric transfer hydrogenation of ketals](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00096D&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 1280-1283
https://doi.org/10.1039/C8QO00096D
Isolation and biosynthesis of labdanmycins: four new labdane diterpenes from endophytic Streptomyces
The gene cluster of two new labdanmycins was identified from an endophytic Streptomyces. The P450 enzyme LabE was confirmed to oxidize C-20 methyl of the biosynthetic intermediate 3 to afford labdanmycins.
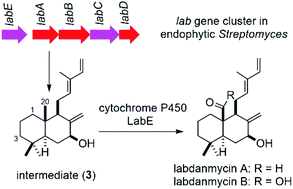
Org. Chem. Front., 2018,5, 1272-1279
https://doi.org/10.1039/C8QO00085A
Base-promoted [3 + 3] cyclization of cyclopropenones and cyclopropenethiones with amides for the synthesis of 6H-1,3-oxazin-6-ones and 6H-1,3-thiazin-6-ones
A facile synthesis of 6H-1,3-oxazin-6-ones and 6H-1,3-thiazin-6-ones has been achieved in the base-promoted [3 + 3] cyclization of cyclopropenones and cyclopropenethiones with amides.
![Graphical abstract: Base-promoted [3 + 3] cyclization of cyclopropenones and cyclopropenethiones with amides for the synthesis of 6H-1,3-oxazin-6-ones and 6H-1,3-thiazin-6-ones](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO00091C&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 1267-1271
https://doi.org/10.1039/C8QO00091C
Copper-catalyzed asymmetric hydroboration of 1,3-enynes with pinacolborane to access chiral allenylboronates
A wide range of functionalized 1,3-enynes undergo hydroboration with pinacolborane to produce enantiomerically enriched allenylboronates in the presence of a chiral copper catalyst.
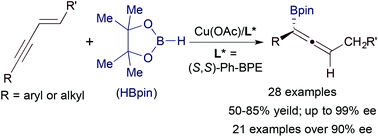
Org. Chem. Front., 2018,5, 1284-1287
https://doi.org/10.1039/C8QO00167G
Isolation, structural elucidation, and synthetic study of salviyunnanone A, an abietane derived diterpenoid with a 7/5/6/3 ring system from Salvia yunnanensis
Salviyunnanone A (1), a cytotoxic diterpenoid possessing an unprecedented architecture was characterized, and its 6-epi-isomer was synthetic achieved in 9 steps.
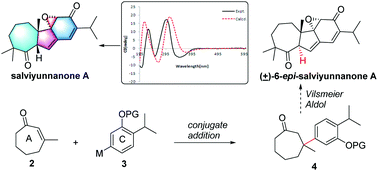
Org. Chem. Front., 2018,5, 1262-1266
https://doi.org/10.1039/C7QO01140G
Syntheses and structures of [7]helicene and double helicene based on dithieno[2,3-b:2′,3′-d]thiophene
Based on dithieno[2,3-b:2′,3′-d]thiophene, three novel helicenes including helicene (rac-1), double helicene (rac-2), and benzohexathia[7]helicene (rac-3), and one bull's horn-shaped benzohexathienoacene (4) have been synthesized and reported in this paper.
![Graphical abstract: Syntheses and structures of [7]helicene and double helicene based on dithieno[2,3-b:2′,3′-d]thiophene](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7QO01049D&imageInfo.ImageIdentifier.Year=2018)
Org. Chem. Front., 2018,5, 1257-1261
https://doi.org/10.1039/C7QO01049D
A general and efficient Mn-catalyzed acceptorless dehydrogenative coupling of alcohols with hydroxides into carboxylates
A general and efficient Mn-catalyzed acceptorless dehydrogenative coupling of alcohols with hydroxides into carboxylates has been developed.

Org. Chem. Front., 2018,5, 1248-1256
https://doi.org/10.1039/C8QO00023A
n Bu4NOTf-promoted radical self-hydrogen transferring isomerization under transition metal-free conditions
We report the first allylic isomerization of alcohols catalyzed by nBu4NOTf generated in situ from tetrabutylammonium triflate and potassium tert-butoxide.
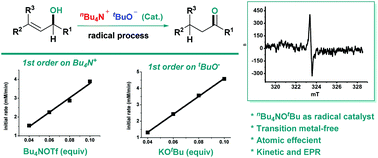
Org. Chem. Front., 2018,5, 1213-1216
https://doi.org/10.1039/C7QO01138E
Synthesis of chiral γ-aminophosphonates through the organocatalytic hydrophosphonylation of azadienes with phosphites
An organocatalytic enantioselective 1,4-addition of phosphites to azadienes has been successfully developed using quinine as a catalyst, providing an efficient and facile route to optically active γ-aminophosphonates with up to 94% ee.
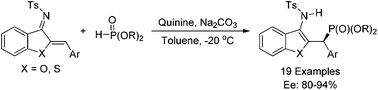
Org. Chem. Front., 2018,5, 1148-1151
https://doi.org/10.1039/C7QO01158J
A concise synthesis of indene-based polycyclic compounds via FeCl3-catalyzed cascade cyclization
An efficient and straightforward protocol to complex indene-based polycyclic compounds via the cascade cyclization of propargylic alcohols and alkenes was developed. The catalyst FeCl3, as an Earth-abundant and environment friendly transition metal salt, is attractive. This reaction proceeded through unusual C–C bond cleavage.
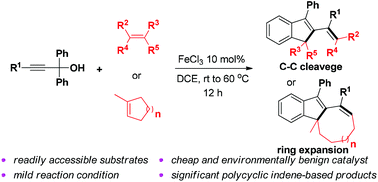
Org. Chem. Front., 2018,5, 1165-1169
https://doi.org/10.1039/C8QO00004B
N-Heterocyclic carbene promoted enantioselective desymmetrization reaction of diarylalkane-bisphenols
An enantioselective NHC-catalyzed desymmetrization reaction of diarylalkane-bisphenols with aldehydes was reported under the guidance of linear free energy relationships (LFERs).
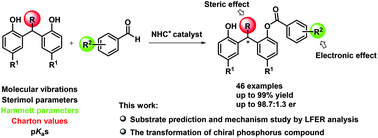
Org. Chem. Front., 2018,5, 1101-1107
https://doi.org/10.1039/C7QO01083D
Iron/zinc-catalyzed benzannulation reactions of 2-(2-oxo-alkyl)benzketones leading to naphthalene and isoquinoline derivatives
An efficient method for the synthesis of poly-substituted naphthalenes/isoquinolines via Fe(III)/Zn(II)-catalyzed [4 + 2] cycloaddition of 2-(2-oxo-alkyl)benzketones is described.
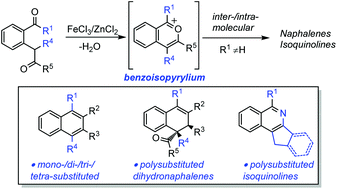
Org. Chem. Front., 2018,5, 1028-1033
https://doi.org/10.1039/C7QO01152K
Racemic trinorsesquiterpenoids from the Beihai sponge Spongia officinalis: structure and biomimetic total synthesis
Two rare new furan butanolides, sponalisolides A (1) and B (2), were isolated from the Beihai sponge Spongia officinalis, and fully characterized by extensive spectroscopic analysis and biomimetic total synthesis.
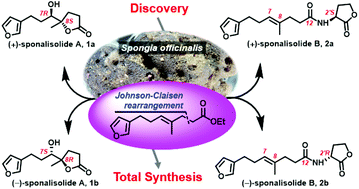
Org. Chem. Front., 2018,5, 1022-1027
https://doi.org/10.1039/C7QO01091E
ortho-Amino group functionalized 2,2′-bipyridine based Ru(II) complex catalysed alkylation of secondary alcohols, nitriles and amines using alcohols
The catalytic activity of Ru(II) complexes in sustainable C–C and C–N bond formation is enhanced by using a functionalized 2,2′-bipyridine ligand.
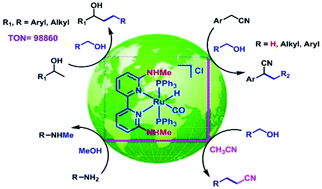
Org. Chem. Front., 2018,5, 1008-1018
https://doi.org/10.1039/C7QO01061C
Rhodium(III)-catalyzed annulative coupling between arenes and sulfoxonium ylides via C–H activation
Sulfoxonium ylides acts as a bifunctional C2-synthon in Rh(III)-catalyzed redox-neutral annulative coupling with arenes for the synthesis of N-heterocycles and carbocycles.
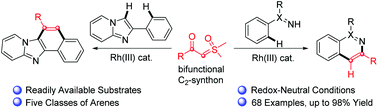
Org. Chem. Front., 2018,5, 998-1002
https://doi.org/10.1039/C7QO01033H
Copper-catalyzed three-component phosphorylation–peroxidation of alkenes
A copper-catalyzed three-component phosphorylation–peroxidation of alkenes with P(O)–H compounds and TBHP has been developed.

Org. Chem. Front., 2018,5, 972-976
https://doi.org/10.1039/C7QO01045A
Radical rearrangement of N-sulfonyl-N-aryl propynamides: proceeding with homolytic N–SO2 bond cleavage and 6-endo-dig cyclization toward 3-sulfonyl-2(1H)-quinolinones
A di-tert-butyl peroxide-promoted radical rearrangement of N-sulfonyl-N-aryl propynamides has been developed, giving 2-sulfonyl-3-aryl-2(1H)-quinolinones in moderate to good yields.

Org. Chem. Front., 2018,5, 958-961
https://doi.org/10.1039/C7QO01048F
Visible-light-induced tandem phosphorylation cyclization of vinyl azides under mild conditions
This method provides a visible-light-induced radical tandem cyclization for the synthesis of phosphorus phenanthridines with various nitrogen-containing substrates.
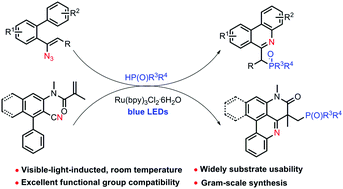
Org. Chem. Front., 2018,5, 822-826
https://doi.org/10.1039/C7QO01004D
The hexameric resorcinarene capsule as an artificial enzyme: ruling the regio and stereochemistry of a 1,3-dipolar cycloaddition between nitrones and unsaturated aldehydes
The hexameric resorcinarene capsule as an artificial enzyme to rule the regio and stereochemistry of a 1,3-dipolar cycloaddition.

Org. Chem. Front., 2018,5, 827-837
https://doi.org/10.1039/C7QO00942A