Azidoheteroarylation of unactivated olefins through distal heteroaryl migration†
Abstract
The radical-mediated azidoheteroarylation of unactivated olefins has been accomplished for the first time based on the strategy of intramolecular distal heteroaryl migration. A variety of synthetically useful heteroaryl-substituted alkyl azides are readily furnished under mild reaction conditions. The utility of the protocol is manifested by readily converting the products to piperidine, β-pipecolic acid, and triazole derivatives.
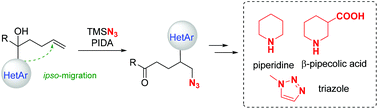
- This article is part of the themed collections: Celebrating the 90th birthday of Professor Lu Xiyan and Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...