Copper-catalyzed asymmetric hydroboration of 1,3-enynes with pinacolborane to access chiral allenylboronates†
Abstract
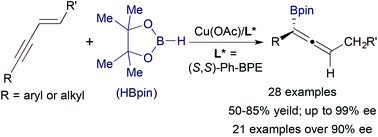
We report a copper-catalyzed enantioselective hydroboration of 1,3-enynes with the catalyst generated from Cu(OAc) and (S,S)-Ph-BPE. A wide range of alkyl- and aryl-substituted 1,3-enynes undergo this asymmetric hydroboration with pinacolborane, yielding the corresponding allenes in good yields with high to excellent enantioselectivities (up to 99% ee). This asymmetric transformation tolerates a variety of reactive groups, such as chloro, bromo, trifluoromethyl ether, siloxy, carboxylic ester and imido functionalities.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...