Synthesis of chiral γ-aminophosphonates through the organocatalytic hydrophosphonylation of azadienes with phosphites†
Abstract
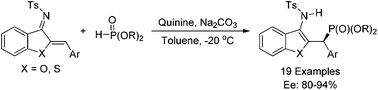
An organocatalytic enantioselective 1,4-addition of phosphites to azadienes has been successfully developed using quinine as a catalyst, providing an efficient and facile route to optically active γ-aminophosphonates with up to 94% ee.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...