The design of rigid cyclic tripyrrins: the importance of intermolecular interactions on aggregation and luminescence†
Abstract
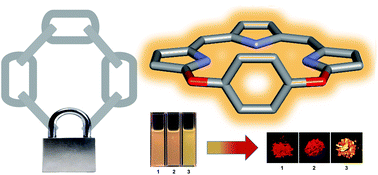
We designed a cyclic tripyrrin “locked” by a bridging benzene-1,4-diol-moiety which is perpendicular to the tripyrrin plane. The cyclic structures promote the scaffold rigidity and enhance the fluorescence. While the halogen-substitution of the bridging moiety was found to slightly affect the fluorescence of 1–3 in solution, such substitution significantly affects the aggregation behaviour and solid emission. 1 and 2 exhibit typical J-aggregate emission but 3 does not. The analysis of crystal packing combined with the reduced density gradient (RDG) analysis suggests that the bridging moieties of 1–3 play an important role in inducing the different crystal packing by tuning intermolecular interactions in the solid state. The energy decomposition analysis using symmetry adapted perturbation theory (SAPT) revealed that such weak intermolecular interactions mainly consist of halogen bonds and π–π interactions.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...