Themed collection New Talent

Artificial supramolecular protein assemblies as functional high-order protein scaffolds
Artificial supramolecular protein assemblies can serve as novel high-order scaffolds that can display various functional proteins with defined valencies and organization, offering unprecedented functional bio-architectures.
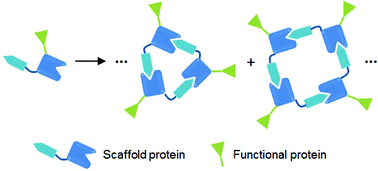
Org. Biomol. Chem., 2016,14, 5352-5356
https://doi.org/10.1039/C6OB00116E
Site-directed spin labeling of proteins for distance measurements in vitro and in cells
We here review strategies for site-directed spin labeling (SDSL) of proteins and discuss their potential for EPR distance measurements to study protein function in vitro and in cells.

Org. Biomol. Chem., 2016,14, 5468-5476
https://doi.org/10.1039/C6OB00473C
Enantioselective polyene cyclizations
A comprehensive review covering the field of enantioselective polyene cyclizations.
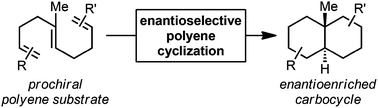
Org. Biomol. Chem., 2016,14, 5454-5467
https://doi.org/10.1039/C6OB00375C
Palladium catalysed meta-C–H functionalization reactions
The directing group assisted site selective C–H functionalization approach is having a continuous impact in the field of natural product synthesis, drug discovery and material sciences.
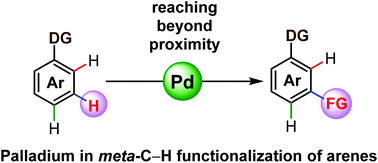
Org. Biomol. Chem., 2016,14, 5440-5453
https://doi.org/10.1039/C6OB00395H
Selective chemical labeling of proteins
Diverse bioorthogonal reactions and chemical tagging approaches for protein labeling are discussed and compared in this review.
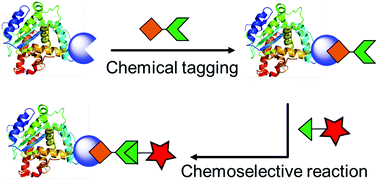
Org. Biomol. Chem., 2016,14, 5417-5439
https://doi.org/10.1039/C6OB00126B
Recent developments in synthetic methods for benzo[b]heteroles
This review summarizes recent developments in synthetic methods for benzoheterole compounds of significant potential for applications in materials science.
![Graphical abstract: Recent developments in synthetic methods for benzo[b]heteroles](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6OB00219F&imageInfo.ImageIdentifier.Year=2016)
Org. Biomol. Chem., 2016,14, 5402-5416
https://doi.org/10.1039/C6OB00219F
Nitropyrrole natural products: isolation, biosynthesis and total synthesis
A review of the isolation, biological activity, biosynthesis and chemical synthesis of nitropyrrole-containing natural products reported to date, including the pyrrolomycins, heronapyrroles and nitropyrrolins.
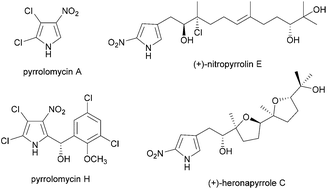
Org. Biomol. Chem., 2016,14, 5390-5401
https://doi.org/10.1039/C5OB02599K
Trihaloethenes as versatile building blocks for organic synthesis
Trihaloethenes are versatile C2-building blocks that can be simply modified via addition, elimination and transition metal-catalyzed reactions.
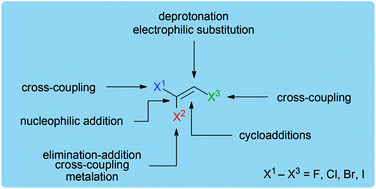
Org. Biomol. Chem., 2016,14, 5377-5389
https://doi.org/10.1039/C6OB00091F
A new generation of chiral phase-transfer catalysts
A new generation of chiral phase-transfer catalysts is summarized.
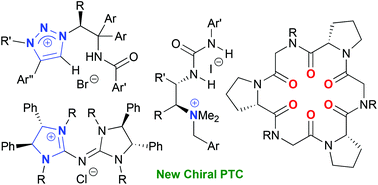
Org. Biomol. Chem., 2016,14, 5367-5376
https://doi.org/10.1039/C5OB02446C
Enantioselective oxidative boron Heck reactions
This review highlights the use of the oxidative boron Heck reaction in enantioselective Heck-type couplings.
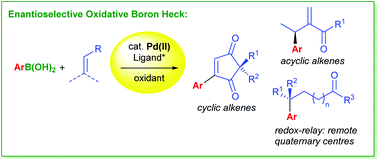
Org. Biomol. Chem., 2016,14, 5357-5366
https://doi.org/10.1039/C5OB01984B
Scalable anti-Markovnikov hydrobromination of aliphatic and aromatic olefins
A simple synthesis of primary bromides from alkenes using HBr in AcOH and atmospheric oxygen as a radical initiator.

Org. Biomol. Chem., 2016,14, 5622-5626
https://doi.org/10.1039/C6OB00692B
Investigation of the dynamic nature of 1,2-oxazines derived from peralkylcyclopentadiene and nitrosocarbonyl species
We have investigated the dynamic nature of 1,2-oxazines, which revealed broad tunability and potential for applications in covalent adaptable materials.
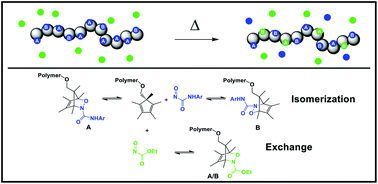
Org. Biomol. Chem., 2016,14, 5617-5621
https://doi.org/10.1039/C6OB00400H
Synthesis of 4H-chromenes by an unexpected, K3PO4-mediated intramolecular Rauhut–Currier type reaction
The K3PO4-mediated intramolecular Rauhut-Currier type reaction of chalcones having an enoate moiety leading to the formation of 4H-chromenes in moderate to good yields is demonstrated.

Org. Biomol. Chem., 2016,14, 5612-5616
https://doi.org/10.1039/C6OB00654J
Protein-specific localization of a rhodamine-based calcium-sensor in living cells
A small synthetic calcium sensor that can be site-specifically coupled to any protein of interest in living cells by utilizing the bio-orthogonal HaloTag labeling strategy.
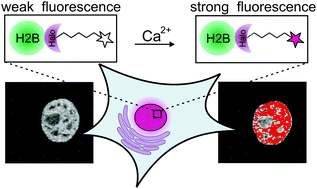
Org. Biomol. Chem., 2016,14, 5606-5611
https://doi.org/10.1039/C6OB00365F
First total synthesis of trehalose containing tetrasaccharides from Mycobacterium smegmatis
Total synthesis of three important trehalose containing tetrasaccharides isolated from Mycobacterium smegmatis is reported for the first time, using regioselective opening of benzylidene acetals and stereoselective glycosylations as key steps.
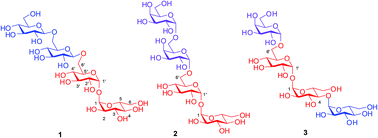
Org. Biomol. Chem., 2016,14, 5595-5598
https://doi.org/10.1039/C6OB00412A
Mechanistic studies on intramolecular C–H trifluoromethoxylation of (hetero)arenes via OCF3-migration
Intramolecular C–H trifluoromethoxylation of arenes and heteroarenes proceeds through a reaction mechanism of radical O-trifluoromethoxylation and ionic OCF3-migration.

Org. Biomol. Chem., 2016,14, 5599-5605
https://doi.org/10.1039/C6OB00132G
Facile synthesis of enantioenriched phenol-sulfoxides and their aluminum complexes
Chiral phenolic p-tolylsulfoxides, t-butylsulfoxides and well-defined aluminum complexes were prepared by short synthetic routes from readily available starting materials.
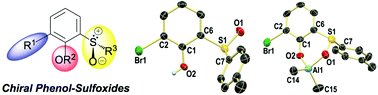
Org. Biomol. Chem., 2016,14, 5580-5585
https://doi.org/10.1039/C6OB00136J
Stereoselective synthesis of thiazino[4,3-a]indoles using the thia-Pictet–Spengler reaction of indoles bearing N-tethered thiols and vinylogous thiocarbonates
Thia-Pictet–Spengler cyclization is used for stereoselective synthesis of N-fused thiazinoindole derivatives. One-pot, sequential Friedel–Crafts alkylation – Pictet–Spengler cyclization and the synthesis of thiazino-oxepino-indole is developed.
![Graphical abstract: Stereoselective synthesis of thiazino[4,3-a]indoles using the thia-Pictet–Spengler reaction of indoles bearing N-tethered thiols and vinylogous thiocarbonates](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6OB00501B&imageInfo.ImageIdentifier.Year=2016)
Org. Biomol. Chem., 2016,14, 5586-5590
https://doi.org/10.1039/C6OB00501B
A mild preparation of alkynes from alkenyl triflates
We report herein a protocol for preparing alkynes from alkenyl triflates at ambient temperature with LiCl as a promoter.
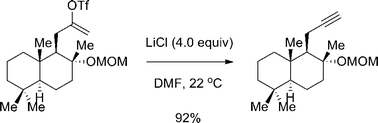
Org. Biomol. Chem., 2016,14, 5591-5594
https://doi.org/10.1039/C6OB00345A
Probing the self-assembly and stability of oligohistidine based rod-like micelles by aggregation induced luminescence
The synthesis and self-assembly of a new C2-symmetric oligohistidine amphiphile equipped with an aggregation induced emission luminophore is reported.
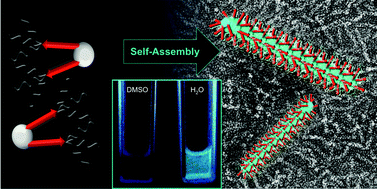
Org. Biomol. Chem., 2016,14, 5574-5579
https://doi.org/10.1039/C6OB00292G
Rhodium-catalyzed pyridannulation of indoles with diazoenals: a direct approach to pyrido[1,2-a]indoles
A new rhodium-catalyzed [4 + 2]-pyridannulation of 3-substituted indoles with diazoenals resulted in the biologically important pyrido[1,2-a]indoles.
![Graphical abstract: Rhodium-catalyzed pyridannulation of indoles with diazoenals: a direct approach to pyrido[1,2-a]indoles](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6OB00360E&imageInfo.ImageIdentifier.Year=2016)
Org. Biomol. Chem., 2016,14, 5569-5573
https://doi.org/10.1039/C6OB00360E
One-pot relay catalysis: divergent synthesis of furo[3,4-b]indoles and cyclopenta[b]indoles from 3-(2-aminophenyl)-1,4-enynols
Described herein a divergent strategy for the synthesis of furo[3,4-b]indoles via a sequential Ag(I)/Bi(III)/Pd(II) catalysis and cyclopenta[b]indoles via a one-pot Ag(I)/Brønsted acid relay catalysis, starting from the easily accessible 3-(2 aminophenyl)-4-pentenyn-3-ols.
![Graphical abstract: One-pot relay catalysis: divergent synthesis of furo[3,4-b]indoles and cyclopenta[b]indoles from 3-(2-aminophenyl)-1,4-enynols](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6OB00319B&imageInfo.ImageIdentifier.Year=2016)
Org. Biomol. Chem., 2016,14, 5563-5568
https://doi.org/10.1039/C6OB00319B
Synthesis of 2-diphospho-myo-inositol 1,3,4,5,6-pentakisphosphate and a photocaged analogue
Diphosphoinositol polyphosphates (inositol pyrophosphates, X-InsP7) are a family of second messengers with important roles in eukaryotic biology. A new approach targeting 2-InsP7 and a photocaged analogue is described.
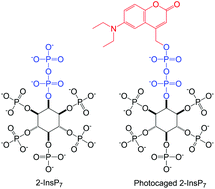
Org. Biomol. Chem., 2016,14, 5559-5562
https://doi.org/10.1039/C6OB00094K
Ruthenium photoredox-triggered phospholipid membrane formation
As more methodologies for generating and manipulating biomimetic cellular systems are developed, opportunities arise for combining different methods to create more complex synthetic biological constructs.
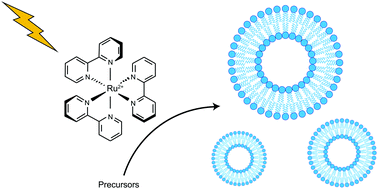
Org. Biomol. Chem., 2016,14, 5555-5558
https://doi.org/10.1039/C6OB00290K
Some chemical speculation on the biosynthesis of corallidictyals A–D
The efficient conversion of siphonodictyal B into the spirocyclic natural products corallidictyals A–D has been achieved via oxidative and acid catalyzed cyclizations.
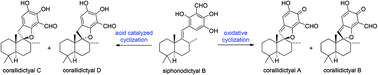
Org. Biomol. Chem., 2016,14, 5546-5549
https://doi.org/10.1039/C6OB00171H
N-Heterocyclic carbene catalysed 1,6-hydrophosphonylation of p-quinone methides and fuchsones: an atom economical route to unsymmetrical diaryl- and triarylmethyl phosphonates
A direct method for the synthesis of diaryl- and triarylmethyl phosphonates through 1,6-hydrophosphonylation of p-quinone methides and fuchsones using NHC as a Brønsted base catalyst is described.
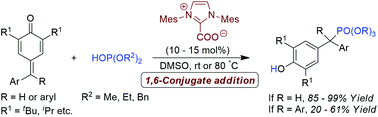
Org. Biomol. Chem., 2016,14, 5550-5554
https://doi.org/10.1039/C6OB00289G
Comparison of boron-assisted oxime and hydrazone formations leads to the discovery of a fluorogenic variant
We use kinetic data, photophysical properties, and mechanistic analyses to compare recently developed high-rate constant oxime and hydrazone formations.
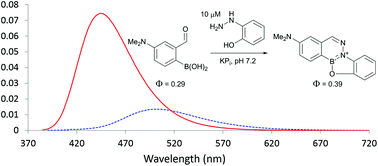
Org. Biomol. Chem., 2016,14, 5529-5533
https://doi.org/10.1039/C6OB00168H
3-Component synthesis of α-substituted sulfonamides via Brønsted acid-catalyzed C(sp3)–H bond functionalization of 2-alkylazaarenes
A Brønsted acid-catalyzed addition of 2-alkylazaarenes to in situ generated N-sulfonylimines through selective C(sp3)–H bond functionalization has been developed.

Org. Biomol. Chem., 2016,14, 5525-5528
https://doi.org/10.1039/C6OB00108D
Phage-displayed macrocyclic glycopeptide libraries
In this report, we describe an efficient way to generate libraries of macrocyclic glycopeptides in one step by reacting phage-displayed libraries of peptides with dichloro-oxime derivatives.
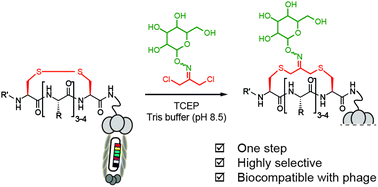
Org. Biomol. Chem., 2016,14, 5539-5545
https://doi.org/10.1039/C5OB02646F
Direct synthesis of anilines and nitrosobenzenes from phenols
A one-pot synthesis of anilines and nitrosobenzenes from phenols has been developed using an ipso-oxidative aromatic substitution (iSOAr) process.
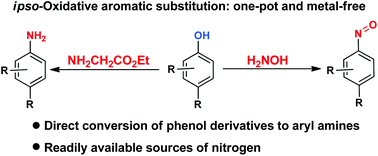
Org. Biomol. Chem., 2016,14, 5520-5524
https://doi.org/10.1039/C6OB00073H
Fluorine-directed 1,2-trans glycosylation of rare sugars
To reconcile the urgent need to access well defined β-configured 2,6-di-deoxypyranose analogues for chemical biology, with the intrinsic α-selectivity of the native system, the directing role of fluorine at C2 has been explored.
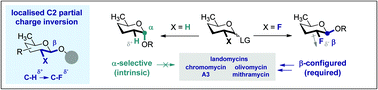
Org. Biomol. Chem., 2016,14, 5534-5538
https://doi.org/10.1039/C6OB00025H
Palladium-catalyzed β-C(sp3)–H arylation of phthaloyl alanine with hindered aryl iodides: synthesis of complex β-aryl α-amino acids
An efficient protocol for palladium-catalyzed PE auxiliary-directed C(sp3)–H arylation reaction with sterically hindered aryl iodides was developed.
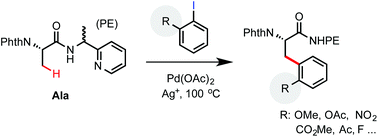
Org. Biomol. Chem., 2016,14, 5511-5515
https://doi.org/10.1039/C5OB02580J
Iron-catalyzed arylation of α-aryl-α-diazoesters
An iron-catalyzed arylation of α-aryl-α-diazoesters with electron-rich benzene rings was developed, which provides an efficient method for the preparation of 1,1-diarylacetates with high yields and excellent chemo- and regio-selectivities.
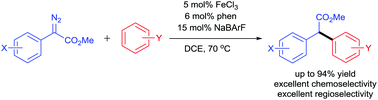
Org. Biomol. Chem., 2016,14, 5516-5519
https://doi.org/10.1039/C5OB02418H
A highly enantioselective Hg(II)-catalyzed Sakurai–Hosomi reaction of isatins with allyltrimethylsilanes
A chiral complex derived from (S)-difluorophos and Hg(OTf)2 is identified as a powerful catalyst for the Sakurai–Hosomi reaction of isatins with allyltrimethylsilane, allowing the facile synthesis of valuable building blocks 3-allyl-3-hydroxyoxindoles in up to 97% ee, with only 0.5–1.0 mol% of catalyst loading.
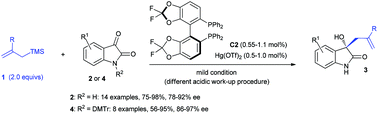
Org. Biomol. Chem., 2016,14, 5500-5504
https://doi.org/10.1039/C5OB02582F
Structure-based design of 3-carboxy-substituted 1,2,3,4-tetrahydroquinolines as inhibitors of myeloid cell leukemia-1 (Mcl-1)
A novel Mcl-1 inhibitor chemotype based on a tetrahydroquinoline carboxylic acid was developed utilizing structure-based design, which was subsequently validated by a fluorescence polarization competition assay and HSQC NMR analysis.
Org. Biomol. Chem., 2016,14, 5505-5510
https://doi.org/10.1039/C5OB02063H
Rawal's catalyst as an effective stimulant for the highly asymmetric Michael addition of β-keto esters to functionally rich nitro-olefins
A general approach to the asymmetric synthesis of highly substituted dihydroquinolines was achieved through neighboring ortho-amino group engaged sequential Michael/amination/dehydration reactions.
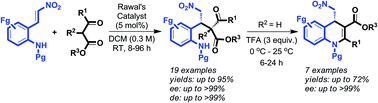
Org. Biomol. Chem., 2016,14, 5494-5499
https://doi.org/10.1039/C5OB02178B
Iron-catalyzed transfer hydrogenation of imines assisted by an iron-based Lewis acid
An iron-catalyzed transfer hydrogenation of N-aryl and N-alkyl imines using isopropanol as the hydrogen donor is reported for the first time.

Org. Biomol. Chem., 2016,14, 5490-5493
https://doi.org/10.1039/C5OB02119G
Copper-catalyzed electrophilic amination using N-methoxyamines
Copper-catalyzed electrophilic amination using functionalized N-methoxyamines, prepared by nucleophilic addition to N-methoxyamides, is reported.
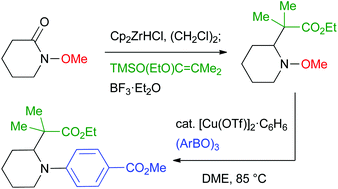
Org. Biomol. Chem., 2016,14, 5486-5489
https://doi.org/10.1039/C5OB02167G
Lewis acid-promoted [2 + 2] cycloadditions of alkenes with aryl ketenes
A method for the [2 + 2] cycloaddition of aryl ketenes and alkenes is presented.
![Graphical abstract: Lewis acid-promoted [2 + 2] cycloadditions of alkenes with aryl ketenes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5OB01837D&imageInfo.ImageIdentifier.Year=2016)
Org. Biomol. Chem., 2016,14, 5477-5480
https://doi.org/10.1039/C5OB01837D
anti-Selective aminofluorination of alkenes with amidines mediated by hypervalent iodine(III) reagents
anti-Aminofluorination of alkenes with amidines was enabled by hypervalent iodine(III) reagents, affording 4-fluoroalkyl-2-imidazolines. Further reductive ring-opening of the 2-imidazoline moiety could deliver highly functionalized 3-fluoropropane-1,2-diamine derivatives.
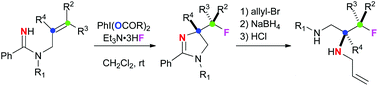
Org. Biomol. Chem., 2016,14, 5481-5485
https://doi.org/10.1039/C5OB01854D
Expedient synthesis of densely substituted pyrrolo[1,2-a]indoles
The strategy was further applied for the synthesis of optically active pyrrolo[1,2-a]indoles in >97% de using the chiral auxiliary based approach.
![Graphical abstract: Expedient synthesis of densely substituted pyrrolo[1,2-a]indoles](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6OB00861E&imageInfo.ImageIdentifier.Year=2016)
Org. Biomol. Chem., 2016,14, 5843-5860
https://doi.org/10.1039/C6OB00861E
A clickable UTP analog for the posttranscriptional chemical labeling and imaging of RNA
A multipurpose UTP analog potentially suitable for RNA aptamer selection and two-channel visualization of RNA in cells by using click chemistry and Raman spectroscopy has been developed.
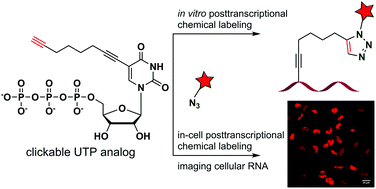
Org. Biomol. Chem., 2016,14, 5832-5842
https://doi.org/10.1039/C6OB00576D
Towards theory driven structure elucidation of complex natural products: mandelalides and coibamide A
The effectiveness of computational tools in determining relative configurations of complex molecules is investigated, using natural products mandelalides A–D and coibamide A, towards a generalized recipe for the scientific community at large.
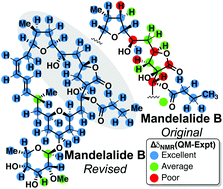
Org. Biomol. Chem., 2016,14, 5826-5831
https://doi.org/10.1039/C6OB00707D
C2-Alkenylation of N-heteroaromatic compounds via Brønsted acid catalysis
C2-alkenylated heteroaromatics can be accessed by simple Brønsted acid catalysed union of diverse heteroarene N-oxides with alkenes. The scope and limitations of the process are outlined.
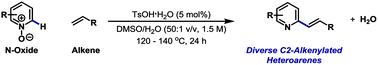
Org. Biomol. Chem., 2016,14, 5820-5825
https://doi.org/10.1039/C6OB00705H
The effect of photoisomerization on the enzymatic hydrolysis of polymeric micelles bearing photo-responsive azobenzene groups at their cores
Photoisomerization boosts the enzymatic disassembly of smart polymeric micelles based on amphiphilic PEG–dendron hybrids bearing enzymatically cleavable azobenzene end-groups.
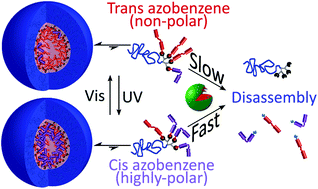
Org. Biomol. Chem., 2016,14, 5813-5819
https://doi.org/10.1039/C6OB00396F
Side-chain-to-tail cyclization of ribosomally derived peptides promoted by aryl and alkyl amino-functionalized unnatural amino acids
A strategy for the production of side-chain-to-tail cyclic peptides from ribosomally derived polypeptide precursors is reported.
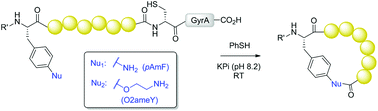
Org. Biomol. Chem., 2016,14, 5803-5812
https://doi.org/10.1039/C6OB00192K
Asymmetric nucleophilic dearomatization of diazarenes by anion-binding catalysis
The first highly regio- and enantioselective nucleophilic dearomatization of diazarenes by anion-binding organocatalysis is reported.
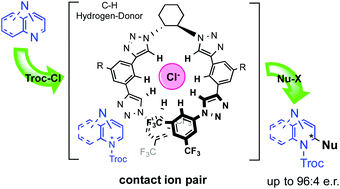
Org. Biomol. Chem., 2016,14, 5794-5802
https://doi.org/10.1039/C6OB00248J
Conformational diversity and enantioconvergence in potato epoxide hydrolase 1
Computational studies highlight the importance of conformational diversity in the enantioconvergent hydrolysis of styrene oxide by potato epoxide hydrolase 1.
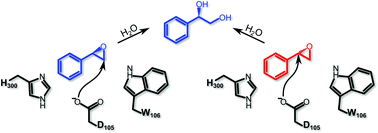
Org. Biomol. Chem., 2016,14, 5639-5651
https://doi.org/10.1039/C6OB00060F
Synthesis of polymers and nanoparticles bearing polystyrene sulfonate brushes for chemokine binding
This paper describes the synthesis of polymers and silica nanoparticles, both bearing polystyrene sulfonate brushes, and the measurement of their binding affinity for the chemokine monocyte chemoattractant protein-1 (MCP-1) in monomeric and dimeric form.
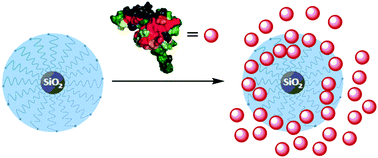
Org. Biomol. Chem., 2016,14, 5652-5658
https://doi.org/10.1039/C6OB00270F
Versatile, mild, and selective reduction of various carbonyl groups using an electron-deficient boron catalyst
Acetanilides and nitriles are mildly and selectively reduced over various other carbonyl derivatives using an electron-deficient boron catalyst and triethylsilane.
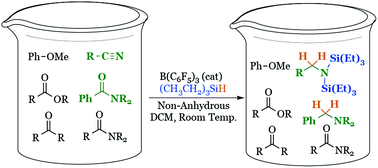
Org. Biomol. Chem., 2016,14, 5774-5778
https://doi.org/10.1039/C6OB00127K
Thermodynamic origin of α-helix stabilization by side-chain cross-links in a small protein
Side-chain cyclization has a consistent effect on protein folding energetics, and the identity of the cross-linking moiety determines the magnitude of stabilization.
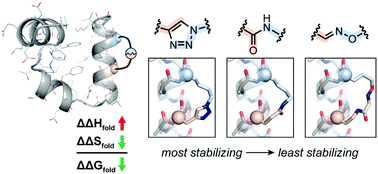
Org. Biomol. Chem., 2016,14, 5768-5773
https://doi.org/10.1039/C6OB00475J
Benzothiazole hydrazones of furylbenzamides preferentially stabilize c-MYC and c-KIT1 promoter G-quadruplex DNAs
The stabilization of G-quadruplex DNA structures by using small molecule ligands having simple structural scaffolds has the potential to be harnessed for developing next generation anticancer agents.
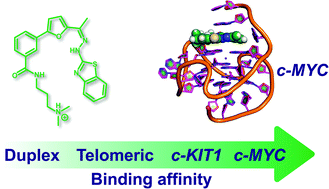
Org. Biomol. Chem., 2016,14, 5779-5793
https://doi.org/10.1039/C6OB00138F
Selective recognition of c-MYC G-quadruplex DNA using prolinamide derivatives
Herein we report the design, synthesis, biophysical and biological evaluation of triazole containing prolinamide derivatives as selective c-MYC G-quadruplex binding ligands.
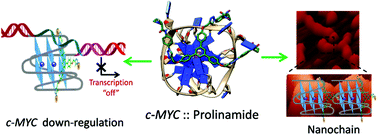
Org. Biomol. Chem., 2016,14, 5761-5767
https://doi.org/10.1039/C6OB00177G
In vivo imaging of advanced glycation end products (AGEs) of albumin: first observations of significantly reduced clearance and liver deposition properties in mice
Molecular imaging visualized significantly reduced clearance of AGE-albumin.

Org. Biomol. Chem., 2016,14, 5755-5760
https://doi.org/10.1039/C6OB00098C
Chiral phosphoric acid catalyzed asymmetric addition of naphthols to para-quinone methides
An asymmetric addition of naphthols to in situ generated para-quinone methides catalyzed by a chiral phosphoric acid is described.
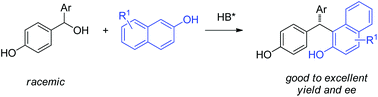
Org. Biomol. Chem., 2016,14, 5751-5754
https://doi.org/10.1039/C6OB00125D
Light-triggered assembly–disassembly of an ordered donor–acceptor π-stack using a photoresponsive dimethyldihydropyrene π-switch
A dimethyldihydropyrene based photochromic π-switch in its closed state forms donor–acceptor stacks with 1,4,5,8-naphthalenetetracarboxylic dianhydride. The stacks collapse in the photoisomeric open form.
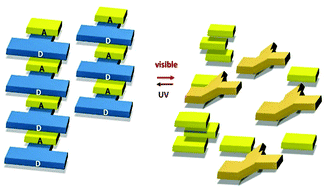
Org. Biomol. Chem., 2016,14, 5744-5750
https://doi.org/10.1039/C6OB00101G
Synthetic studies towards putative yuremamine using an iterative C(sp3)–H arylation strategy
An overview of synthetic efforts toward the initially proposed structure of yuremamine using iterative C(sp3)–H arylations is described.
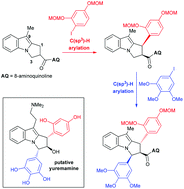
Org. Biomol. Chem., 2016,14, 5728-5743
https://doi.org/10.1039/C6OB00110F
Palladium-catalyzed Suzuki–Miyaura coupling of amides by carbon–nitrogen cleavage: general strategy for amide N–C bond activation
A unified strategy for the palladium-catalyzed Suzuki–Miyaura cross-coupling of amides with boronic acids for the synthesis of ketones by N–C bond activation is reported.
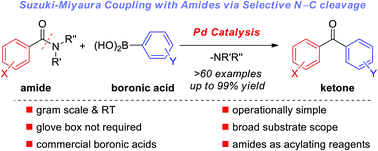
Org. Biomol. Chem., 2016,14, 5690-5707
https://doi.org/10.1039/C6OB00084C
Reversible morphological changes of assembled supramolecular amphiphiles triggered by pH-modulated host–guest interactions
Acid–base modulated host–guest binding at the micellar–water interface triggers reversible oblate ellipsoid-to-lamellar morphological transitions revealing the relationship between 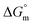 and morphology.
and morphology.
Org. Biomol. Chem., 2016,14, 5714-5720
https://doi.org/10.1039/C6OB00109B
Green organocatalytic α-hydroxylation of ketones
An efficient and green method for the α-hydroxylation of substituted ketones has been developed.
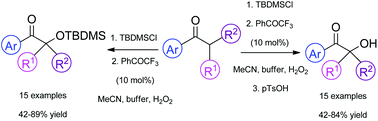
Org. Biomol. Chem., 2016,14, 5708-5713
https://doi.org/10.1039/C6OB00036C
Synthesis and characterization of a highly strained donor–acceptor nanohoop
A highly-strained, nitrogen-doped cycloparaphenylene (CPP), aza[6]CPP, was synthesized and then converted to a donor–acceptor nanohoop, N-methylaza[6]CPP, via alkylation of the nitrogen center.
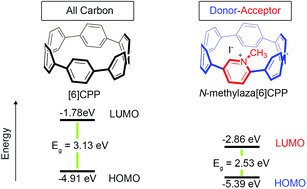
Org. Biomol. Chem., 2016,14, 5721-5727
https://doi.org/10.1039/C6OB00133E
I2 mediated synthesis of 5-substituted-3-methyl/benzyl-1,3,4-oxadiazol-2(3H)-ones via sequential condensation/oxidative cyclization and rearrangement
A simple and efficient iodine-assisted protocol for synthesis of 5-substituted-3-methyl/benzyl-1,3,4-oxadiazol-2(3H)-ones has been developed.
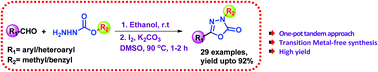
Org. Biomol. Chem., 2016,14, 5683-5689
https://doi.org/10.1039/C5OB02667A
Direct conjugate alkylation of α,β-unsaturated carbonyls by TiIII-catalysed reductive umpolung of simple activated alkenes
The title reaction leads to 1,6-difunctionalized products without the requirement of premetallated reagents. Details on scope, selectivity and mechanism are reported.

Org. Biomol. Chem., 2016,14, 5673-5682
https://doi.org/10.1039/C5OB02631H
A convenient domino Ferrier rearrangement-intramolecular cyclization for the synthesis of novel benzopyran-fused pyranoquinolines
Lewis acid-mediated three component coupling of salicyladehydes, anilines and gylcals provided novel benzopyran-fused pyranoquinolines stereoselectively, in moderate to high yields.
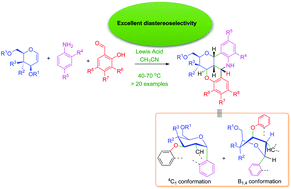
Org. Biomol. Chem., 2016,14, 5627-5638
https://doi.org/10.1039/C5OB02536B
A versatile synthesis of chiral β-aminophosphines
A protocol for the synthesis of chiral β-aminophosphines by ring-opening of cyclic sulfamidates with metallated secondary phosphine oxides is described. It allows access to previously unknown derivatives having electron-deficient, electron-rich and sterically hindered P-aryl substituents.
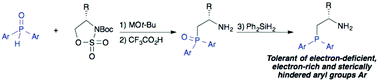
Org. Biomol. Chem., 2016,14, 5665-5672
https://doi.org/10.1039/C5OB02439K
Catalytic asymmetric formal γ-allylation of deconjugated butenolides
A formal γ-allylation of deconjugated butenolides is reported based on a two-step sequence consisting of a catalytic diastereo- and enantioselective vinylogous nucleophilic addition to vinyl sulfones and Julia–Kocienski olefination. This highly modular approach delivers densely functionalized butenolides containing a quaternary stereogenic centre in excellent yield with high enantioselectivity.
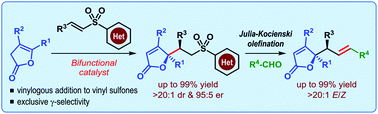
Org. Biomol. Chem., 2016,14, 5659-5664
https://doi.org/10.1039/C5OB02362A
About this collection
This issue aims to highlight the excellent work being carried out by newer members of the research community.
It will cover topics including organic synthesis, physical organic chemistry, supramolecular chemistry and chemical biology, and showcase the strength of research being carried out by tomorrow's leaders in the field.
Articles in this themed issue will be added below as soon as possible after they are published. Please return to this page frequently to see the collection grow.