N-Heterocyclic carbene catalysed 1,6-hydrophosphonylation of p-quinone methides and fuchsones: an atom economical route to unsymmetrical diaryl- and triarylmethyl phosphonates†
Abstract
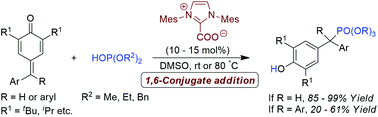
A convenient organocatalytic approach to access unsymmetrical diaryl- and triarylmethyl phosphonates using NHC as a Brønsted base catalyst is described. This atom-economical protocol enables the installation of phosphonate groups on p-quinone methides and fuchsones through a 1,6-conjugate addition of dialkylphosphites, and the corresponding phosphonates were obtained in excellent yields.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...