Versatile, mild, and selective reduction of various carbonyl groups using an electron-deficient boron catalyst†
Abstract
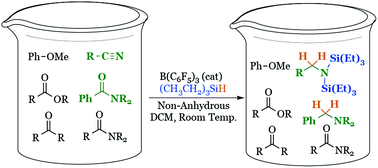
A mild and selective new method was discovered to reduce acetanilides and other carbonyl compounds. Unlike sodium borohydride, which is selective in reducing aldehydes and ketones, this new protocol is uniquely selective in reducing acetanilides and nitriles over other carbonyl containing functional groups. Additionally, β-ketoamides were shown to be reduced at the ketone preferentially over the amide.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...