Synthetic studies towards putative yuremamine using an iterative C(sp3)–H arylation strategy†
Abstract
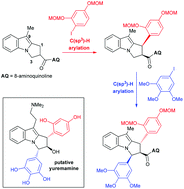
An overview of an iterative, 8-aminoquinoline (AQ)-directed C(sp3)–H arylation strategy towards the pyrroloindole structure initially assigned to the alkaloid yuremamine is described. During initial efforts using a model indane system, it was discovered that the iodoresorcinol unit was not a viable C(sp3)–H arylation partner when masked as its dimethyl ether but upon switching to a MOM group, the ether oxygen served to stabilise the high valent Pd intermediate during the reaction, thus promoting reductive elimination and leading to acceptable yields of the C(sp3)–H arylation product. The second C(sp3)–H arylation with an iodopyrogallol gave a 1,3-diarylated model yuremamine system possessing the desired 1,3-cis relationship. When the successful model studies were applied to a pyrroloindole system in pursuit of yuremamine, it became apparent that C9 underwent competing C(sp2)–H arylation if left vacant, but installing a tryptamine side chain at this site prevented the desired C(sp3)–H arylation from occurring altogether. However, a C9-methyl pyrroloindole underwent iterative C(sp3)–H arylation at C1 with an iodoresorcinol followed by C3 with an iodopyrogallol to give a diarylated product with the aryl groups in the undesired 1,3-trans-relationship, arising from epimerisation at C1 during the second C(sp3)–H arylation event. Although the synthesis of putative yuremamine was not accomplished, several findings are disclosed that will serve as useful additions to the burgeoning field of directed C(sp3)–H arylations and related C–H functionalization reactions.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...