Rawal's catalyst as an effective stimulant for the highly asymmetric Michael addition of β-keto esters to functionally rich nitro-olefins†
Abstract
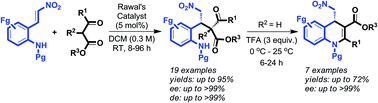
A general approach to asymmetric synthesis of highly substituted dihydroquinolines was achieved through neighboring ortho-amino group engaged sequential Michael/amination/dehydration reactions on (E)-2-(2-nitrovinyl)anilines with cyclic and acyclic β-keto esters in the presence of a catalytic amount of Rawal's quinidine-NH-benzyl squaramide followed by TFA.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...