Investigation of the dynamic nature of 1,2-oxazines derived from peralkylcyclopentadiene and nitrosocarbonyl species†
Abstract
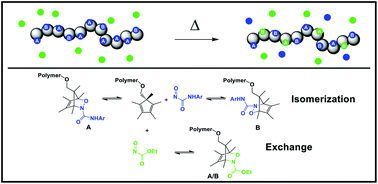
We have investigated the reversible hetero-Diels–Alder reaction of 1,2-oxazines derived from a peralkylcyclopentadiene and a series of nitrosocarbonyl dienophiles. The nature of the dienophile was found to impart broad tunability to the dynamic character of the oxazine adducts. The reversibility was also observed in polymeric systems. The fidelity of the reaction and tunable sensitivity toward elevated temperature and water signify potential applications in the development of dynamic covalent materials or delivery systems for small molecule payloads.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...