Asymmetric nucleophilic dearomatization of diazarenes by anion-binding catalysis†
Abstract
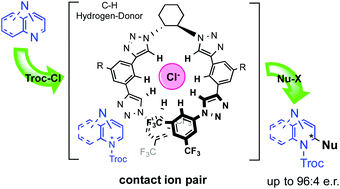
The first anion-binding organocatalyzed enantioselective Reissert-type dearomatization of diazarenes has been developed. This reaction represents a synthetic challenge since diazarenes have various reactive sites. The use of a chiral tetrakistriazole as a C–H-based hydrogen-donor catalyst allowed the straightforward highly regio- and enantioselective synthesis of a variety of chiral diazaheterocycles.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...