Trihaloethenes as versatile building blocks for organic synthesis
Abstract
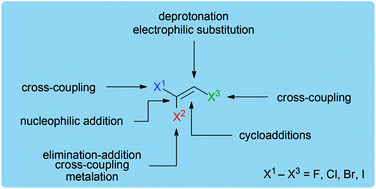
This review highlights the chemistry of trihaloethene building blocks with a special focus on commercially available 1,1,2-trichloroethene. The topics surveyed herein include the use of trihaloethenes as C2-building blocks for transition metal-catalyzed coupling reactions, addition, elimination and cycloaddition reactions as well as natural product syntheses.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...