Synthesis and characterization of a highly strained donor–acceptor nanohoop†
Abstract
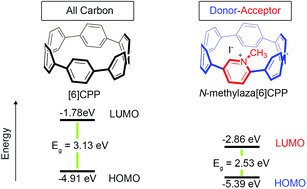
A highly-strained, nitrogen-doped cycloparaphenylene (CPP), aza[6]CPP, was synthesized and then converted to a donor–acceptor nanohoop, N-methylaza[6]CPP, via alkylation of the nitrogen center. The energy levels of the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) for both molecules were then probed by cyclic voltammetry (CV), which revealed that the donor–acceptor nanohoop had a significantly lower LUMO energy relative to [6]CPP and aza[6]CPP. Density functional theory (DFT) revealed that the donor–acceptor nanohoop underwent a redistribution of the frontier molecular orbital (FMO) density such that a significant portion of the LUMO density resided upon the electron-deficient nitrogen-containing ring. This localization of LUMO density caused a large lowering in the LUMO energy of nearly a full electron volt, while the HOMO energy was less affected due to a large centralization of the FMO on the electron-rich phenylene backbone. This ultimately resulted in a net lowering of the HOMO–LUMO energy gap which was observed both experimentally and computationally. In addition, N-methylaza[6]CPP has a significantly lower energy LUMO than N-methylaza[8]CPP, illustrating that the FMO levels of donor–acceptor nanohoops can be tuned by adjusting the hoop size.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...