Themed collection Organic chemist’s toolbox

Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: a review
Recently, quinoline has become an essential heterocyclic compound due to its versatile applications in the fields of industrial and synthetic organic chemistry.
RSC Adv., 2020,10, 20784-20793
https://doi.org/10.1039/D0RA03763J
Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry
Nitrogen containing heterocycles are of immense research interest because they are often found as naturally occurring bioactive compounds.
RSC Adv., 2020,10, 14170-14197
https://doi.org/10.1039/D0RA01378A
CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery: review
The review lays emphasis on the significance of 1,2,3-triazoles synthesized via CuAAC reaction having potential to act as anti-microbial, anti-cancer, anti-viral, anti-inflammatory, anti-tuberculosis, anti-diabetic, and anti-Alzheimer drugs.

RSC Adv., 2020,10, 5610-5635
https://doi.org/10.1039/C9RA09510A
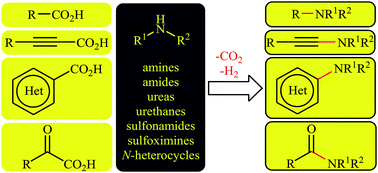
Recent developments in decarboxylative cross-coupling reactions between carboxylic acids and N–H compounds
Carboxylic acids and their derivatives are ubiquitous compounds in organic chemistry, and are widely commercially available in a large structural variety.
RSC Adv., 2019,9, 8964-8976
https://doi.org/10.1039/C9RA00929A
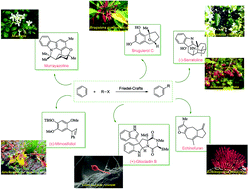
Applications of Friedel–Crafts reactions in total synthesis of natural products
In this review, we try to underscore the applications of intermolecular and intramolecular FC reactions in the total syntheses of natural products and complex molecules, exhibiting diverse biological properties.
RSC Adv., 2018,8, 40061-40163
https://doi.org/10.1039/C8RA07325B
Beyond a solvent: triple roles of dimethylformamide in organic chemistry
N,N-Dimethylformamide (DMF) is frequently used as an aprotic solvent in chemical transformations. It is a multipurpose compound besides being an effective polar aprotic solvent. DMF can be act as a reagent, a catalyst and a stabilizer.

RSC Adv., 2018,8, 27832-27862
https://doi.org/10.1039/C8RA04985H
Combining transition metals and transient directing groups for C–H functionalizations
This review is about the momentous evolution of transient directing group assisted C–H functionalizations promoted by three major second row transition metals.
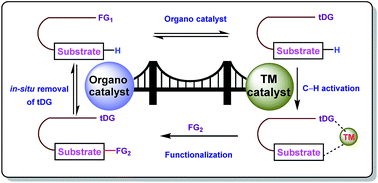
RSC Adv., 2018,8, 19456-19464
https://doi.org/10.1039/C8RA03230K
Recent advances in the application of indoles in multicomponent reactions
An overview on recent applications of indoles in multicomponent reactions for the synthesis of heterocyclic compounds is provided.
RSC Adv., 2018,8, 12069-12103
https://doi.org/10.1039/C7RA13321A
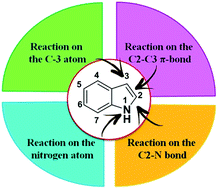
Recent advances in the chemistry of 2-chloroquinoline-3-carbaldehyde and related analogs
This review describes the recent publications reported on the chemistry of 2-chloroquinoline-3-carbaldehydes. Heterocyclic quinoline ring systems are binary and fused cycles.
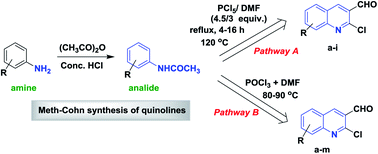
RSC Adv., 2018,8, 8484-8515
https://doi.org/10.1039/C7RA11537G
Total synthesis of natural products containing benzofuran rings
In this review, various approaches for the construction of benzofurans as an important moiety in different natural products during the total synthesis of the natural of products are underscored.

RSC Adv., 2017,7, 24470-24521
https://doi.org/10.1039/C7RA03551A
Metal-free domino one-pot protocols for quinoline synthesis
Metal-free domino one-pot protocols for quinoline synthesis have been reviewed.
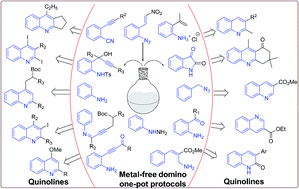
RSC Adv., 2015,5, 42020-42053
https://doi.org/10.1039/C5RA07798B
Hydrosilylation reaction of olefins: recent advances and perspectives
This review focuses on the recent development of efficient, selective, and cheaper hydrosilylation catalyst systems appearing in the last decade.
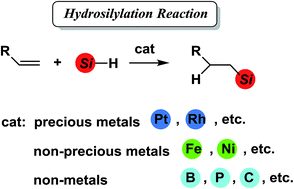
RSC Adv., 2015,5, 20603-20616
https://doi.org/10.1039/C4RA17281G
Pyrrole: a resourceful small molecule in key medicinal hetero-aromatics
Pyrrole is widely known as a biologically active scaffold which possesses a diverse nature of activities.
RSC Adv., 2015,5, 15233-15266
https://doi.org/10.1039/C4RA15710A
Part I: Nitroalkenes in the synthesis of heterocyclic compounds
The applications of nitroalkenes in the synthesis of three- to five-membered O, N and S-heterocycles, including natural products are investigated in this review.

RSC Adv., 2014,4, 48022-48084
https://doi.org/10.1039/C4RA08828J
Recent advances in the synthesis of quinolines: a review
This review article gives information about the recent advances in the synthesis of quinoline derivatives by various eco-friendly, green and clean protocols.
RSC Adv., 2014,4, 24463-24476
https://doi.org/10.1039/C4RA01814A
Glymes as versatile solvents for chemical reactions and processes: from the laboratory to industry
Glymes are versatile solvents/regents that have wide applications in many research areas and commercial products.
RSC Adv., 2014,4, 11251-11287
https://doi.org/10.1039/C3RA47191H
Recent developments in palladium catalysed carbonylation reactions
Carbonylation reactions with a special emphasis on catalyst–product separation techniques are reviewed and discussed.
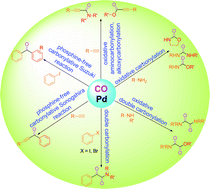
RSC Adv., 2014,4, 10367-10389
https://doi.org/10.1039/C3RA46273K
Dual protection of amino functions involving Boc
This review deals with the synthesis, properties and applications of acyl- (1) and sulfonylcarbamates (2) and imido- (3) and acylimidodicarbonates (4), obtained on N-Boc-protection of amides and carbamates, with particular attention to facilitated cleavage of well-known amino-protecting groups. Compounds of type 1 and 2 are easily cleaved, 3 alkylated and cleaved and 4 reduced and transaminated.
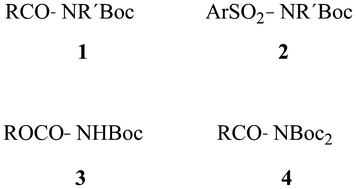
RSC Adv., 2013,3, 18691-18697
https://doi.org/10.1039/C3RA42956C
Transition metal-catalyzed decarboxylative coupling reactions of alkynyl carboxylic acids
The decarboxylative coupling of alkynyl carboxylic acids is an attractive area of research in organic chemistry, because the structure of aryl alkyne is one of the important building blocks for the synthesis of p-conjugated compounds.
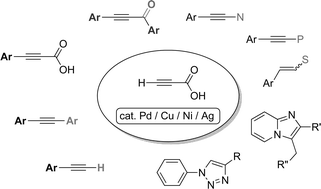
RSC Adv., 2013,3, 14165-14182
https://doi.org/10.1039/C3RA41442F
Recent developments in solvent-free multicomponent reactions: a perfect synergy for eco-compatible organic synthesis
This review reports the development of multicomponent reactions over the last 10 years.
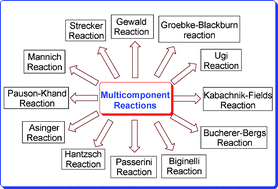
RSC Adv., 2012,2, 4547-4592
https://doi.org/10.1039/C2RA01056A
Homogeneous and heterogeneous catalysts for multicomponent reactions
A number of multicomponent reactions, homogeneous and heterogeneous acid and base catalyzed, are overviewed.
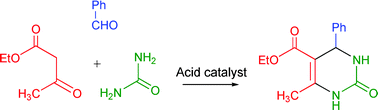
RSC Adv., 2012,2, 16-58
https://doi.org/10.1039/C1RA00807B
Iodine-mediated synthesis of (E)-vinyl sulfones from sodium sulfinates and cinnamic acids in aqueous medium
A green and highly efficient method for the synthesis of (E)-vinyl sulfones promoted by iodine in water has been developed, without transition metal catalysts and ligands.
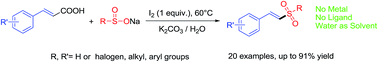
RSC Adv., 2015,5, 66723-66726
https://doi.org/10.1039/C5RA10896A
Iodonium salts as efficient iodine(III)-based noncovalent organocatalysts for Knorr-type reactions
The dibenziodolium cation displays high catalytic activity for the Knorr-type reactions via binding with the carbonyl O atom.
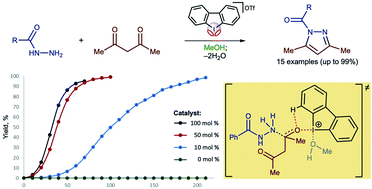
RSC Adv., 2021,11, 4574-4583
https://doi.org/10.1039/D0RA09640G
Small organic molecules with tailored structures: initiators in the transition-metal-free C–H arylation of unactivated arenes
A small organic molecule was tailored for the efficient synthesis of biphenyl and its derivatives from aryl iodides.

RSC Adv., 2020,10, 14500-14509
https://doi.org/10.1039/D0RA01845G
Synthesis of 1,3-diaryl-spiro[azetidine-2,3′-indoline]-2′,4-diones via the Staudinger reaction: cis- or trans-diastereoselectivity with different addition modes
Two experimental techniques of the ketene–imine Staudinger reaction allowed different diastereomers of spiro-indolinone-β-lactams to be obtained.
![Graphical abstract: Synthesis of 1,3-diaryl-spiro[azetidine-2,3′-indoline]-2′,4-diones via the Staudinger reaction: cis- or trans-diastereoselectivity with different addition modes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0RA02374D&imageInfo.ImageIdentifier.Year=2020)
RSC Adv., 2020,10, 14122-14133
https://doi.org/10.1039/D0RA02374D
An umpolung reaction of α-iminothioesters possessing a cyclopropyl group
Tandem N-alkylation/oxidation/second addition reaction to α-cyclopropyl α-imino(thio)esters gave N-alkylated ring-opened products in good yields.

RSC Adv., 2020,10, 9955-9963
https://doi.org/10.1039/D0RA01152E
An intramolecular relay catalysis strategy for Knoevenagel condensation and 1,3-dipolar cycloaddition domino reactions
A magnetically recoverable bifunctional catalyst was synthesized and effectively used in Knoevenagel condensation and 1,3-dipolar cycloaddition domino reactions.
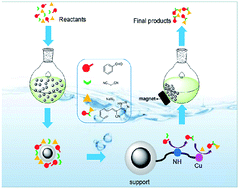
RSC Adv., 2019,9, 23614-23621
https://doi.org/10.1039/C9RA04081A
Double nucleophilic addition to iminomalonate, leading to the synthesis of quaternary α-amino diesters and desymmetrization of the products
Alkylation of iminomalonate with Grignard reagents followed by oxidation and allylation gave symmetrical quaternary α-amino diesters. Subsequent desymmetrization of a diol derivative from these products was conducted via asymmetric carbamylation catalyzed by Cu-Bnbox.

RSC Adv., 2019,9, 23400-23407
https://doi.org/10.1039/C9RA04889H
Facile one-pot synthesis of sulfonyl fluorides from sulfonates or sulfonic acids
A mild one-pot protocol for directly converting sulfonates or sulfonic acids into sulfonyl fluorides was developed.

RSC Adv., 2019,9, 13863-13867
https://doi.org/10.1039/C9RA02531F
An iodine/DMSO-catalyzed sequential one-pot approach to 2,4,5-trisubstituted-1H-imidazoles from α-methylene ketones
A sequential one-pot approach to 2,4,5-trisubstituted imidazoles has been developed from α-methylene ketones and aldehydes.
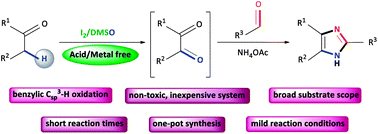
RSC Adv., 2018,8, 37557-37563
https://doi.org/10.1039/C8RA07238H
Development of a metal-free amine oxidation method utilizing DEAD chemistry
Development of a metal-free methodology for the efficient and general dehydrogenation of amines, accomplished by employing azodicarboxylate as oxidizing agents.
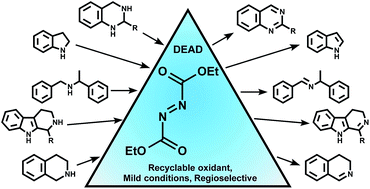
RSC Adv., 2017,7, 48848-48852
https://doi.org/10.1039/C7RA09165F
K2CO3-promoted aerobic oxidative cross-coupling of trialkyl phosphites with thiophenols
Phosphorylation of thiols has been achieved via K2CO3-promoted aerobic oxidative cross-coupling of trialkyl phosphites with thiophenols.
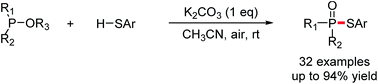
RSC Adv., 2017,7, 45416-45419
https://doi.org/10.1039/C7RA09057A
Simple and efficient Fmoc removal in ionic liquid
The combination of triethylamine and [Bmim][BF4] represents a mild method for efficient removal of the Fmoc group.
RSC Adv., 2017,7, 36482-36491
https://doi.org/10.1039/C7RA04425A
Regioselective thiolation of electron rich arenes and heterocycles in recyclable catalytic media
A convenient and novel approach has been developed for the synthesis of unsymmetrical diaryl sulfides by the reaction of sulfonyl hydrazides with phenols, aromatic amines and heterocycles using a [Bmim][Br] ionic liquid through C–S bonds formation.
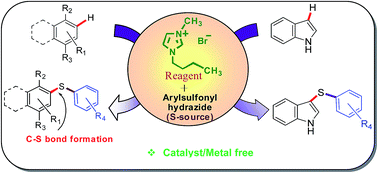
RSC Adv., 2017,7, 22860-22868
https://doi.org/10.1039/C7RA02350B
Efficient esterification of n-butanol with acetic acid catalyzed by the Brönsted acidic ionic liquids: influence of acidity
BAILs having different structures and their related acidities have been investigated for their role in the esterification of n-butanol with acetic acid, and it was found that IL-5 containing double –SO3H groups exhibits excellent catalytic activity.
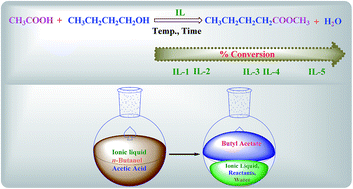
RSC Adv., 2017,7, 5412-5420
https://doi.org/10.1039/C6RA26722J
TBAI–HBr system mediated generation of various thioethers with benzenesulfonyl chlorides in PEG400
An efficient procedure was developed for the synthesis of aryl sulfides in good to excellent yields using TBAI–HBr system promoted direct sulfenylation of various compounds, such as phenols, pyrazolones, indoles and related heteroarenes.
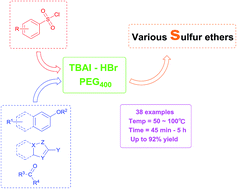
RSC Adv., 2016,6, 54377-54381
https://doi.org/10.1039/C6RA02302A
Novel trinitroethanol derivatives: high energetic 2-(2,2,2-trinitroethoxy)-1,3,5-triazines
The multicomponent reaction of 2,4,6-trichloro-1,3,5-triazine with potassium trinitromethane and trinitroethanol was exploited for the first synthesis of the hetaryl trinitroethyl ethers.
RSC Adv., 2016,6, 34921-34934
https://doi.org/10.1039/C6RA05826D
A rapid metal free synthesis of 5-substituted-1H-tetrazoles using cuttlebone as a natural high effective and low cost heterogeneous catalyst
A convenient, rapid and metal free synthesis of 5-substituted-1H-tetrazoles by [3 + 2] cycloaddition reaction of nitriles with sodium azide. Cuttlebone as a natural low cost heterogeneous catalyst affords 5-substituted-1H-tetrazoles rapidly with high efficiency.
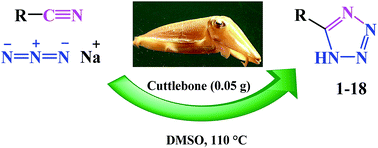
RSC Adv., 2015,5, 49849-49860
https://doi.org/10.1039/C5RA08147E
Iron(II) bromide-catalyzed oxidative coupling of benzylamines with ortho-substituted anilines: synthesis of 1,3-benzazoles
An iron(II) bromide-catalyzed oxidative coupling of benzylamines with 2-amino/hydroxy/mercapto-anilines has been developed, allowing the synthesis of a diversity of substituted 1,3-benzazoles in good to excellent yields.
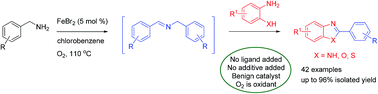
RSC Adv., 2015,5, 5015-5023
https://doi.org/10.1039/C4RA12490A
Cs2CO3 promoted direct C–H bond sulfenylation of imidazo[1,2-a]pyridines and related heteroarenes in ionic liquid
Cs2CO3-promoted sulfenylation of imidazo[1,2-a]pyridines in ionic liquid.
![Graphical abstract: Cs2CO3 promoted direct C–H bond sulfenylation of imidazo[1,2-a]pyridines and related heteroarenes in ionic liquid](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4RA01240B&imageInfo.ImageIdentifier.Year=2014)
RSC Adv., 2014,4, 19891-19895
https://doi.org/10.1039/C4RA01240B
Oxidative synthesis of quinazolinones and benzothiadiazine 1,1-dioxides from 2-aminobenzamide and 2-aminobenzenesulfonamide with benzyl alcohols and aldehydes
An interesting procedure for the zinc-catalyzed oxidative transformation of ready available 2-aminobenzamide, 2-aminobenzenesulfonamide with benzyl alcohols has been developed.
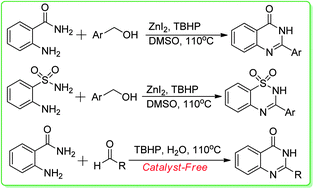
RSC Adv., 2014,4, 8-17
https://doi.org/10.1039/C3RA45765F
General and efficient method for direct N-monomethylation of aromatic primary amines with methanol
The direct N-monomethylation of aromatic primary amines, including arylamines, arylsulfonamides and amino-azoles, using methanol as a methylating agent has been accomplished in the presence of a [Cp*IrCl2]2/NaOH system.
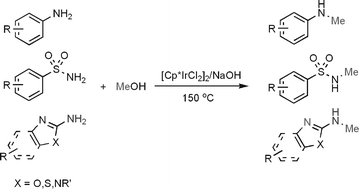
RSC Adv., 2012,2, 8645-8652
https://doi.org/10.1039/C2RA21487C
About this collection
We are very pleased to present our 10th Anniversary collection on organic chemist’s toolbox!Looking back over the last 10 years, we would like to showcase some of the very best articles that have been published in RSC Advances. Many of these papers have been cited hundreds of times, providing valuable advances for further research, and some continue to be among the journal’s most downloaded articles as of today.
We hope you enjoy our 10th Anniversary collection on organic chemist’s toolbox!