Oxidative synthesis of quinazolinones and benzothiadiazine 1,1-dioxides from 2-aminobenzamide and 2-aminobenzenesulfonamide with benzyl alcohols and aldehydes†
Abstract
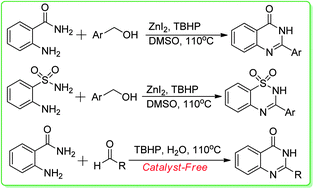
An interesting procedure for the zinc-catalyzed oxidative transformation of ready available 2-aminobenzamide, 2-aminobenzenesulfonamide with benzyl alcohols has been developed. Various quinazolinones and benzothiadiazine 1,1-dioxides were prepared in moderate to good yields under identical conditions. The reactions of both aromatic aldehydes and aliphatic aldehydes with 2-aminobenzamide under catalyst free conditions were described as well. In water media, the products were formed in good yields.
- This article is part of the themed collections: Organic chemist’s toolbox and Organic chemistry collection

 Please wait while we load your content...
Please wait while we load your content...