Transition metal-catalyzed decarboxylative coupling reactions of alkynyl carboxylic acids
Abstract
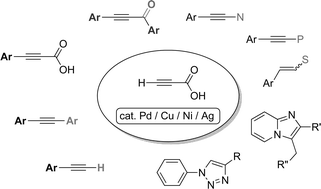
The decarboxylative coupling of alkynyl carboxylic acids is an attractive area of research in organic chemistry, because the structure of aryl alkyne is one of the important building blocks for the synthesis of p-conjugated compounds. The use of alkynyl carboxylic acid as an alkyne source has several advantages in handling and storage. As a catalyst, palladium, copper, nickel, and silver were employed in the decarboxylative coupling reactions. The formation of C–C (sp2–sp, sp–sp and sp3–sp), C–N, C–P and C–S bonds has been developed. This review aims to illustrate the development of the decarboxylative coupling reaction of alkynyl carboxylic acids.
- This article is part of the themed collection: Organic chemist’s toolbox

 Please wait while we load your content...
Please wait while we load your content...