K2CO3-promoted aerobic oxidative cross-coupling of trialkyl phosphites with thiophenols†
Abstract
Convenient, practical and economical phosphorylation of thiols has been achieved via halogen- and metal-free K2CO3-promoted aerobic oxidative cross-coupling of trialkyl phosphites, dimethyl phenylphosphonite, or methyl diphenylphosphinite with thiophenols using air as the oxidant at room temperature. This transformation provides a straightforward route to the construction of phosphorus–sulfur bonds with wide functional group compatibility, which affords phosphorothioates in up to 94% yield.
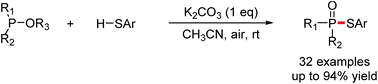
- This article is part of the themed collection: Organic chemist’s toolbox


 Please wait while we load your content...
Please wait while we load your content...