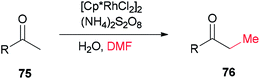Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Beyond a solvent: triple roles of dimethylformamide in organic chemistry
Majid M. Heravi
 *,
Mahdieh Ghavidel
and
Leyla Mohammadkhani
*,
Mahdieh Ghavidel
and
Leyla Mohammadkhani

Department of Chemistry, School of Sciences, Alzahra University, Vanak, Tehran, Iran. E-mail: mmh1331@yahoo.com; mmheravi@alzahra.ac.ir
First published on 3rd August 2018
Abstract
N,N-Dimethylformamide (DMF) is frequently used as an aprotic solvent in chemical transformations in laboratories of academia as well as in those of chemical industry. In the present review, we will reveal that DMF is actually something much more than a solvent. It is a unique chemical since, as well as being an effective polar aprotic solvent, it can play three other important roles in organic chemistry. It can be used as a reagent, a catalyst, and a stabilizer.
1. Introduction
N,N-Dimethylformamide (DMF) is an extraordinary organic compound with the formula (CH3)2NC(O)H. It generally abbreviated and literally always called, DMF (should not be mistaken with dimethylfuran or dimethyl fumarate). It is a colourless liquid with a high boiling point which is miscible with water and also with a majority of common organic solvents. DMF should be naturally odorless, however in its technical grade or degraded samples every so often it has a fishy smell due to some impurity of dimethylamine.1,2As its name implies, it is a formamide derivative. Although, among most chemists, DMF is known as an inexpensive commercially available solvent which is widely used in synthetic organic chemistry, by a second look and further consideration, it is actually more than a solvent, showing multipurpose applications. It is a polar aprotic, but hydrophilic, solvent that dissolves most organic compounds. Thus, it is especially useful in organic reactions when their mechanisms involve polar species, such as SN2 reactions.3 Therefore, DMF is mostly used as an excellent polar solvent with a relatively low evaporation rate, useful for a wide variety of organic transformations. Its usefulness and superiority as a solvent is due to its favored dissolution actually provided by favorited interactions with a substrate.
This uniquely versatile and powerful chemical is frequently used as a solvent for preparation of colloids,4–6 synthesis of block-copolymers,7,8 and also many other types of organic reactions9 like solvent-free hydrolysis, allylation, decarboxylation, polymerization, etc.10–15 It is also found as a perfect solvent for peptide coupling in the pharmaceuticals industry, in development and production of pesticides and in the manufacture of adhesives, synthetic leathers, fibers, films, and surface coatings. It also is used in the manufacturing of dyes as an important raw material which is consumed during a reaction.16 DMF also is a common solvent utilized in electrospinning.17,18
Beyond being used as an useful solvent, DMF is employed as a reagent in some important organic reactions, such as the Vilsmeier–Haack reaction,19 which results in the formylation of aromatic, non-aromatic, and heteroaromatic compounds.19–30 DMF can also be used as a reagent in two other important organic name reactions such as the Friedel–Crafts reaction31 and Beckmann rearrangement.32,33
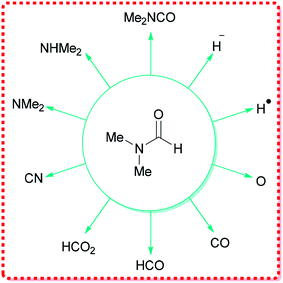
DMF can react as either an electrophilic or a nucleophilic agent, thus, can be considered as the source of various key intermediates mediating a plethora of important reactions.34 More significantly, because of its structure, DMF can participate in many reactions by serving as a multipurpose building block for various units, such as HCO2, O, CO, H˙, H−, NMe2, CONMe2, Me, CHO, etc. (Fig. 1).
It is also a common catalyst35–38 used in the synthesis of acyl halides, in particular, and in the synthesis of acyl chloride from carboxylic acids using oxalyl or thionyl chloride. The catalytic mechanism entails reversible formation of an imidoyl chloride.39,40 It acts as a catalyst in preparing the corresponding azepine,12 for the cycloaddition reaction of CO2 to propylene oxide,41,42 synthesis of cyclic carbonates,43 condensation reactions of alkylisocyanides (or arylisocyanides) with barbituric acid derivatives,13 reductive ring-cleavage of isoxazole,10 allylation of aldehydes,44 conversion of both primary and secondary alcohols to bromides,45 and acylation of aromatic compounds.46
It was disclosed that DMF also can act as a stabilizer in some reactions. In this regard, synthesis of DMF-stabilized metal nanoclusters and nanoparticles were reported.47–51 Copper (Cu),52 iridium (Ir),53 gold (Au),54 palladium (Pd),55 silver (Ag),56 and Fe2O3 (ref. 57) nanoparticles are stabilized with DMF molecules through interaction of amido groups of DMF with NCs.
In addition, DMF usually serves as a solvent in the formation of peptide-bonds.58 DMF dissolves amino acids and coupling reagents and does not react with piperidine, but this reagent can jeopardize peptide synthesis by decomposition into formaldehyde (HCHO) and dimethylamine (HNMe2).59
Worthy of mention is that on the basis of published reports by NIOSH (The National Institute for Occupational Safety and Health), DMF is readily absorbed through the skin or by inhalation or ingestion. This chemical is a potent liver toxin. DMF is also known to cause constipation, abdominal pain, vomiting and nausea, headache, weakness, dizziness, skin problems, and alcohol intolerance.60
The solvent effect of DMF on the efficiency and solvation of anions was reviewed by Parker in 1962.61 Other significant functions of DMF, such as being utilized as a ligand, reducing agent, and dehydrating agent were reported by Muzart and co-workers in 2009.34 In addition, recent developments in the applications of DMF in the area of nitrilation, amination, formylation, aminocarbonylation, and amidation, as well as its reaction with arynes was reviewed in 2012;62 thus, these are not deliberated herein. As a matter of fact, the goal of this report is to disclose other roles of this unique chemical as reagent, catalyst, and stabilizer which have not been comprehensively reviewed. Herein, we try to provide an accessible reference and attract the attention of chemists and stir up their interest in using DMF in organic synthesis and trying to open other new gateway for more applications of this extraordinary compound as a unique and multipurpose chemical. We also frequently used DMF as an efficient solvent in our laboratory.63–65
Being thoughtful about this remarkable chemical, in this report we focus on the applicability of DMF as reagent, catalyst, and stabilizer.
2. DMF as a reagent
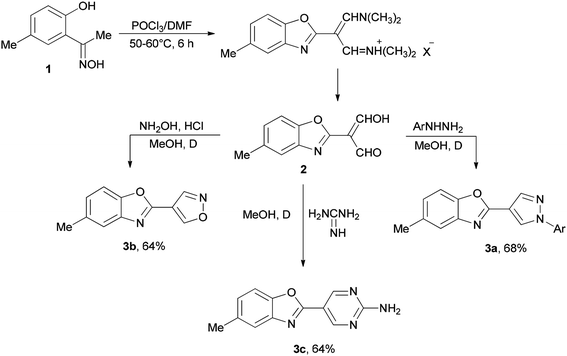
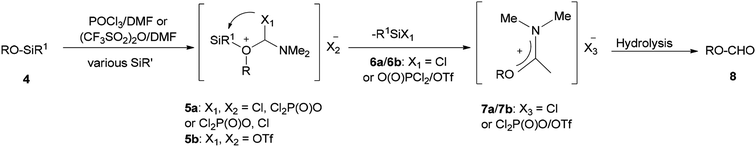
Initially, in 1973, Vilsmeier–Haack reagent (POCl3/DMF) was used for the formation of malondialdehyde 2 from 2-hydroxy-5-methylacetophenone oxime 1 through Beckmann rearrangement followed by cyclization. Kumar and co-workers in 2010 achieved and reported the synthesis of a series of novel heterocycles 3a–3c from compound 2 as a key intermediate (Scheme 1).19 The condensation reaction of 2 with suitable reagents afforded heterocyclic derivatives 3a–3c.66In 1999, Lellouche and Koeller presented an efficient and convenient methodology for preparation of formate esters 8 from O-TBDMS/O-TES protected alcohols 4 (R alkyl residue) under Vilsmeier–Haack reaction conditions in a one-step fashion.67 A plausible mechanism is proposed and described in Scheme 2. In the first step; the POCl3/DMF complex ((CF3SO2)2O/DMF complex) is added to the silyl ether 4 to furnish the oxonium cation 5a (or 5b). In the second step; the formation of Si–Cl/Si–O strong bonds eliminates the R1Si–X1 6a/6b (R1Si = TES or TBDMS) from the resulting intermediate 5a (or 5b) to produce in situ the relevant imidate salts 7a (or 7b) as a mixture of counter anions. The production of the desired formate esters 8 is completed by smooth hydrolysis of 7a (or 7b).
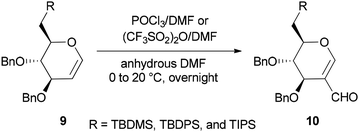
Synthesis of the corresponding C(6)-O-formates 10 was accomplished and reported by the same group in 2001 through a one-step and selective transformation of O-TBDMS, O-TBDPS, and O-TIPS ethers of D-glucal 9, using two electrophilic Vilsmeier–Haack reagents POCl3/DMF or (CF3SO2)2O/DMF in anhydrous DMF at 0–20 °C (Scheme 3).68
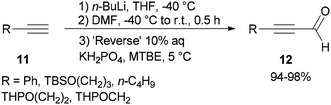
Synthesis of α,β-acetylenic aldehydes 12 was commenced with acetylides 11 which were initially converted to lithium acetylides in the presence of n-BuLi. The formylation of lithium acetylides was accomplished in the presence of DMF with subsequent quenching of the α-aminoalkoxide using 10% aqueous KH2PO4 to provide 12 (>94%) as sole products (Scheme 4).69
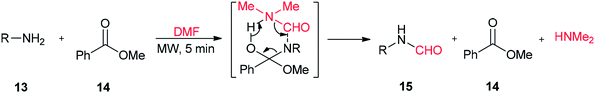
A highly convenient and efficient method was developed by Jeon and Yang for N-formylation of various primary and secondary amines using DMF as a formylating agent in the presence of a catalytic amount of methyl benzoate as a promoter (Scheme 5).70 The main advantages of this methodology are short reaction time, high product yields, neutral reaction conditions, and selective N-formylation in the presence of a hydroxyl group.
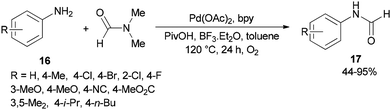
Gu and Guo developed a new protocol for efficient production of N-arylcarboxamides 17 through transamidation of aniline derivatives 16 with dimethylformamide in the presence of a catalytic amount of Pd(OAc)2, 2,2′-bipyridine, PivOH and BF3·Et2O as additives in toluene at 120 °C under O2 atmosphere (Scheme 6).71 This methodology opens a new way for the synthesis of various corresponding transamidation products from commercially available dimethylformamides and anilines.
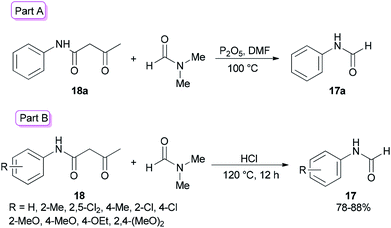
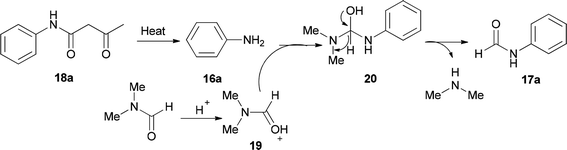
Amides and their derivatives as organic materials are very important in organic synthesis.72,73 They are widely used for preparing many medicines and biologically active compounds.74,75 A novel protocol, including the sequence of a carbon–nitrogen bond formation of β-keto amides with N,N-dimethylamides with subsequent two carbon–nitrogen bonds cleavage using HCl, was achieved by Chen and co-workers in 2015 for the construction of a wide range of R-benzyl formamides and acetamides as well as for the synthesis of various different acyl and aryl R-phenyl amide compounds.76 N-Phenylformamide 17a was produced by reaction of acetoacetanilide 18a with DMF in the presence of P2O5 as the catalyst at 100 °C (Scheme 7, part A). Another novel protocol for the production of N-phenylamides 17 using dimethylformamide and dimethylacetamides 18 as the acyl donors was also developed. The acetoacetanilide derivatives 18 were reacted with DMF under optimal conditions (using HCl in DMF at 120 °C for 12 h) to furnish their corresponding N-phenylformamides 17 (Scheme 7, part B).
As shown in Scheme 8, in the first step, the intermediates 19 and 16a were obtained by protonation of DMF and decomposition of 18a, respectively. Next, compound 20 was produced by nucleophilic attack of 16a on the carbonyl carbon leading to the product 17a by elimination of one equivalent of NHMe2 from 19.
Cyanoindoles are valuable intermediates in organic chemistry. They also are widely used in industry for the generation of pharmaceuticals, agrochemicals, and dyes.77–82 Because of their importance in organic synthesis, numerous methods for their formation have been developed.83–89
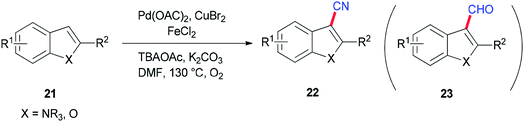
Ding and Jiao in 2011 reported an alternative pathway to generate the aryl nitriles 22 via new and direct Pd-catalyzed cyanation of indoles and benzofurans by C–H bond functionalization using DMF both as a source of CN and as a solvent (Scheme 9).90 Isotopic labeling experiments showed that nitrogen and carbon of the cyano group are generated from DMF.
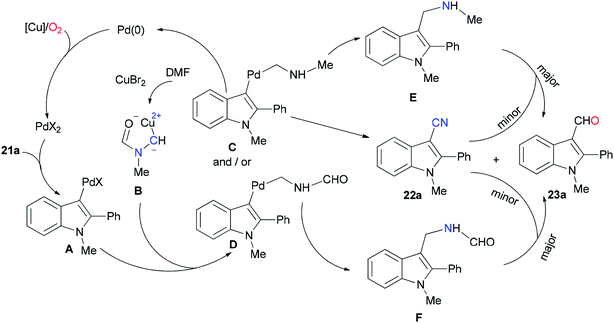
As shown in Scheme 10, the Pd(0) intermediate A is formed by electrophilic aromatic palladation which undergoes an electrophilic reaction with B to give the intermediates C and/or D. The intermediates E and/or F are obtained by reductive elimination of C or D, respectively. The catalytic cycle is completed by reoxidization of the formed Pd(0) complex to Pd(II) by employing O2 and/or Cu(II) salts. Likely, the cyano product 22a is produced either directly from active C or through E as the intermediate, in situ. On the other hand, aldehyde 23a can be formed by oxidation of intermediate F.
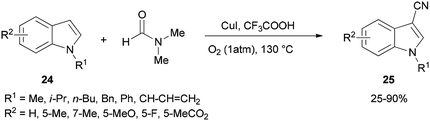
Cheap and safe DMF as a CN source and as a solvent was applied in 2015 by Chen and Han for synthesis of the corresponding C3-cyanoindoles 25 via direct and selective copper-mediated C3-cyanation of indole C–H bonds under an oxygen atmosphere (Scheme 11). A series of control experiments were performed to clarify the source of the ‘CN’ unit. There is no doubt that the nitrogen and carbon atoms come from DMF.91
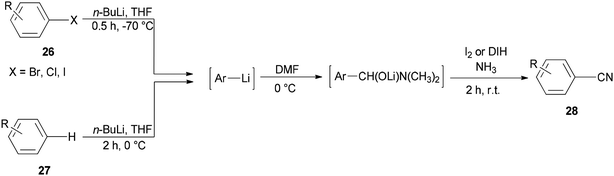
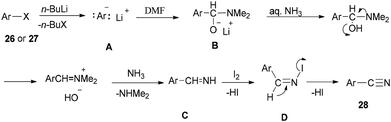
Togo and co-workers generated the corresponding aromatic nitriles 28 by treatment of various aromatic halides 26 and various aromatic compounds 27 in the presence of n-BuLi and then DMF, followed by reaction with I2 in aq NH3 (Scheme 12).92
Scheme 13 shows a plausible reaction mechanism for production of the aromatic nitriles 28. First, aromatic halide 26, or any aromatic compound 27, is reacted with n-BuLi to afford aryllithium A. The second step is formation of the adduct B by treatment of A with DMF. N-Iodo aromatic imine D is yielded via addition of I2 or DIH (1,3-diiodo-5,5-dimethylhydantoin), and aq NH3, followed by reaction with I2, to afford the aromatic nitrile 28 through HI elimination.
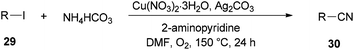
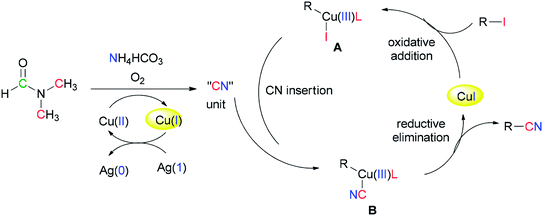
Successfully, Chang and Pawar in 2014 employed DMF and ammonium bicarbonate as a combined source of “CN” units for the Cu(II)-catalyzed cyanation of electron-rich and fused aryl iodides 29 (Scheme 14).93
A proposed mechanism for this catalytic cyanation is illustrated in Scheme 15. With respect to the key roles of copper(II) species in the in situ production of “CN” units and subsequent cyanation of aryl halides, Ag2CO3 re-oxidizes the resultant copper(I) species under copper-mediated oxidative conditions.
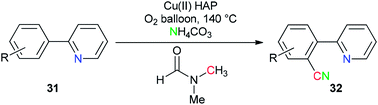
Copper(II) oxide supported on hydroxyapatite [HAP:Ca5(PO4)3(OH)] was used by Venugopal's group as a selective and active catalyst for generation of ‘CN’ units from NH4HCO3 and DMF for the synthesis of aromatic nitriles 32 by safe cyanation of C–H bonds of heteroaryl compounds 31 (Scheme 16).94
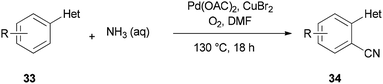
A unique pathway for synthesis of monosubstituted nitriles 34 via regioselectivity cyanation at arene C–H bonds using DMF and NH3 as a combined source of “CN” unit was presented in 2010 by Kim and Chang (Scheme 17).95 Isotopic incorporation experiments indicated that the C and N of the “CN” are derived from the N,N-dimethyl moiety of DMF and NH3, respectively.
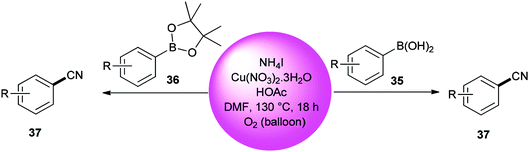
Kim and co-workers used DMF (source of carbon atom) and NH4I (source of nitrogen and iodide atoms) in cyanation of aromatic boronic acids 35 and boronate esters 36 under Cu-mediated oxidative conditions (Scheme 18).95 The reaction is perceived to proceed in a two-step process: iodination and cyanation.
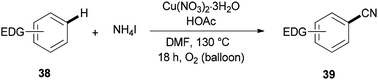
Cyanation of benzenes 38 bearing electron-rich groups on the phenyl ring would be probable because of their talent to perform the initial iodination step. Cyanation of arene C–H bonds was accomplished for the first time by using NH4I and DMF under oxidative copper-mediated conditions (Scheme 19).95
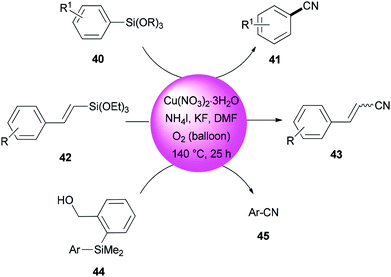
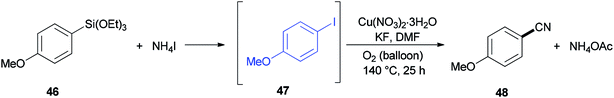
In 2013, DMF and iodide as a combined source of a “CN” unit were used by Wang and Chang for cyanation of organosilanes, such as arylsilanes 40, vinylsilanes 42, and Hiyama silanes 44 under copper-mediated oxidative conditions (Scheme 20).96 As a proposal, the reaction proceeds through two sequential steps: the initial transformation of organosilanes to their corresponding iodo intermediates followed by conventional cyanation.
A reaction profile for cyanation of triethoxy(4-methoxyphenyl)silane 46 was presented under the same reaction conditions (Scheme 21). It commenced with 46, which was transformed almost quantitatively to 4-methoxyiodobenzene 47. Then, with the disappearance of 47, formation of 4-methoxybenzonitrile 48 was started. As a matter of fact, when 48 was subjected to reaction conditions, 47 was provided in 74% yield even in the absence of potassium fluoride, proposing that iodoarenes act as a key intermediate in this cyanation process.
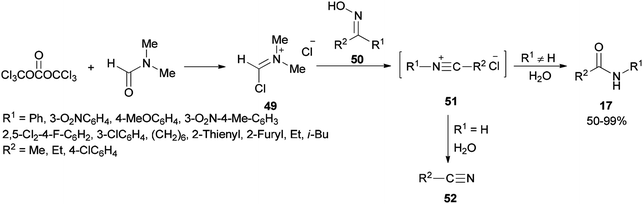
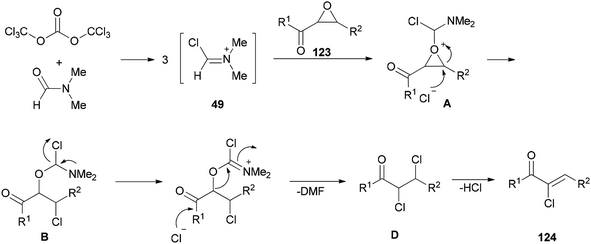
Preparation of amides from ketoximes is known as the Beckmann rearrangement which is generally carried out at high temperatures and in the presence of large amounts of strong acid. This method is applied both in organic chemistry and chemical manufacturing.97–104 Hence, development of a simple, and affordable catalytic system for the Beckmann rearrangement was desired. Su and co-workers synthesized amides 17 via Beckmann rearrangement of oximes 50 using Vilsmeier salt reagent [Me2N+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl] 49 which was produced from treatment of bis-(trichloromethyl) carbonate (BTC) with DMF in refluxing acetonitrile (Scheme 22).105 The fact that the reaction yield was reduced in the absence of DMF or an excess of DMF implies that without using DMF, no Vilsmeier reagent was formed and in excess of DMF, a less reactive adduct was formed. Su's group also synthesized nitriles 52 from intermediates 51.
CHCl] 49 which was produced from treatment of bis-(trichloromethyl) carbonate (BTC) with DMF in refluxing acetonitrile (Scheme 22).105 The fact that the reaction yield was reduced in the absence of DMF or an excess of DMF implies that without using DMF, no Vilsmeier reagent was formed and in excess of DMF, a less reactive adduct was formed. Su's group also synthesized nitriles 52 from intermediates 51.
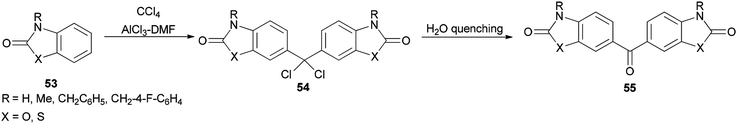
In 1997, Ucar and co-workers achieved and reported the synthesis of a series of symmetrical benzophenone derivatives 55 as single compounds by regioselective Friedel–Crafts C-alkylation reaction of 2(3H)-benzoxazolone and 2(3H)-benzothiazolone with carbon tetrachloride (CCl4) by use of AlCl3-DMF reagent (Scheme 23).106
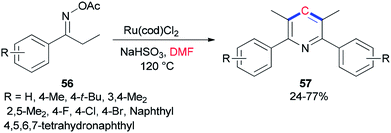
Guan and co-workers prepared tetra-substituted symmetrical pyridines 57 via cyclization of ketoxime carboxylates 56 with N,N-dimethylformamide catalyzed by ruthenium under mild reaction conditions (Scheme 24).107 On the basis of deuterium-labeling experiments accomplished by Guan's group, the carbon unit is produced by a methyl carbon on DMF as a source of one carbon synthon.
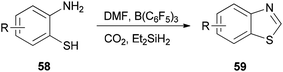
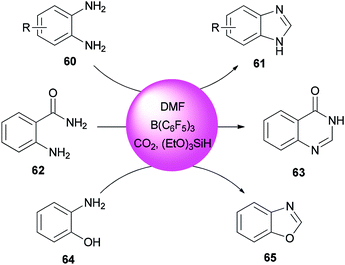
Heterocycles containing nitrogen are widely employed as pharmaceutical and agrochemical agents.108–110 They also extensively act as antibacterial, antiviral, antitubercular, anticancer, antihistaminic, anti-inflammatory, and antihypertensive agents as well as plant growth regulators.111–116 As a new metal-free catalytic system, generation of a series of heterocycles containing nitrogen including benzothiazoles 59, benzomidazoles 61, quinazolinone 63, and benzoxazole 65 were achieved and reported through cyclization of ortho-substituted aniline derivatives with DMF using B(C6F5)3 along with CO2 (Schemes 25 and 26).117
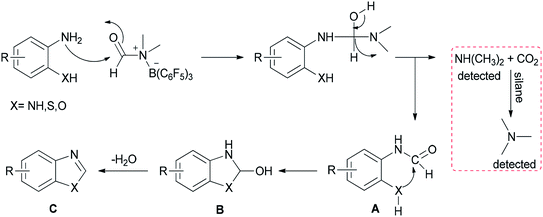
A mechanistic proposal for this reaction is outlined in Scheme 27. First, B(C6F5)3 activates DMF through electrostatic interaction. Next, formylated intermediate A provided by nucleophilic attack of substrates undergoes intramolecular nucleophilic cyclization and loss of H2O to yield products C. Simultaneously, released dimethylamine is treated with CO2 and silane to give the trimethylamine product which pushes the reaction to the right; as a result, the reaction is greatly promoted to proceed to completion.
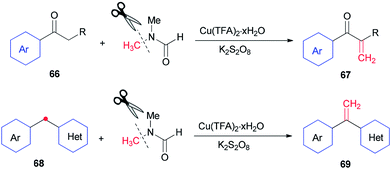
In 2014, Lei and co-workers reported a simple method for the synthesis of terminal olefins including arylvinylketones 67 and arylvinylpyridines 69 by Cu-catalyzed direct oxidative Csp3–H methylenation of arylketones 66 and 1-aryl-1-pyridinemethanes 68, respectively, using DMF as one the sources of carbon and as a solvent (Scheme 28).118 Investigation of a preliminary mechanism shows that CH2 comes from DMF (N–CH3).
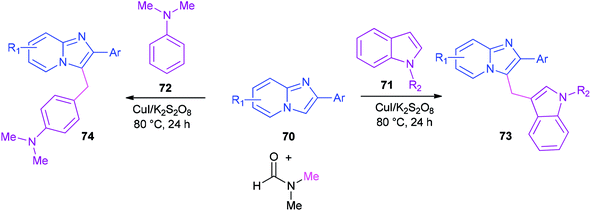
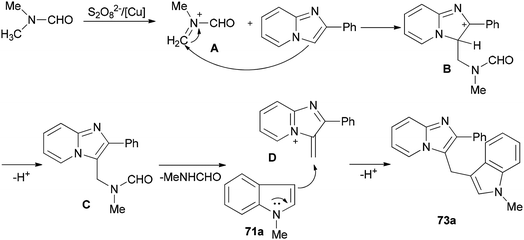
Syntheses of heterodiarylmethanes 73 and 74 was achieved by Hajra and co-workers in 2016 through a copper-catalyzed coupling reaction of imidazo[1,2-a]pyridines 70 using DMF as a methylenating reagent (Scheme 29).119 A series of 3-(1H-indol-3-ylmethyl)-imidazo[1,2-a]pyridine derivatives 73 were prepared under aerobic reaction conditions. This method is also appropriate to prepare (4-imidazo[1,2-a]pyridin-3-ylmethyl)(phenyl)dimethylamines 74. The current methodology opens a new way for synthesis of important building blocks of the heterodiarylmethanes. A possible mechanism for this reaction was proposed (Scheme 30).119
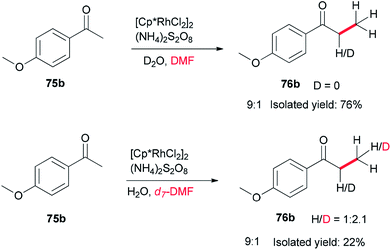
Xue and co-workers in 2014 presented a highly effective protocol which allows rhodium-catalyzed direct methylation of ketones 75 using DMF (Scheme 31).120 Mechanistic studies indicate that the DMF plays dual roles: as the source of carbon for methylation and source of hydrogen for the rhodium-catalyzed reduction of the methylene into a methyl group.
To gain insight into the reaction mechanism, deuterium labeling experiments were accomplished. Replacing H2O with D2O did not significantly affect the product yield. When DMF was exchanged to d7-DMF, the reaction became significantly slower. Thus, it is suggested that the newly formed methyl group is derived from DMF due to a H/D ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1 observed on the newly assembled methyl group (Scheme 32).
2.1 observed on the newly assembled methyl group (Scheme 32).
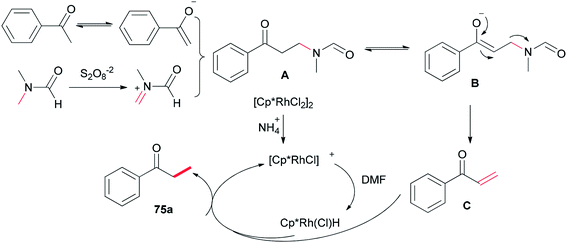
A probable mechanism was proposed which is illustrated in Scheme 33. Persulfate oxidizes DMF to give an iminium intermediate. The intermediate A generated by attack of enolate is converted to intermediate B followed by C–N bond cleavage to create unsaturated ketone intermediate C. Next, the latter is reduced through a Rh–H complex, which is probably created from dehydrogenation of DMF by [Cp*RhCl2]2, leading to the methylated product 75a, with the transfer process a methyl group from DMF to the ketone. In the reduction step, (NH4)2S2O8 can help the dissociation of Cl from Rh(III) through hydrogen bonding with its NH4+ ion.
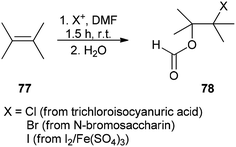
The corresponding β-haloformates 78 were generated in high regio- and stereoselectivity by treatment of alkenes 77 with DMF in the presence of sources of electrophilic halogens (trichloroisocyanuric acid or N-bromosaccharin or I2/Fe2(SO4)3) followed by addition of water (Scheme 34).121
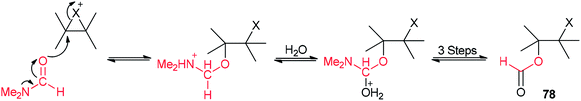
A mechanistic scheme for this transformation is shown in Scheme 35. The halonium ion intermediate is converted to an iminium ion by nucleophilic attack of the oxygen of DMF. Then, the hydrolysis of iminium ion yields the β-haloformate.
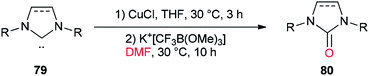
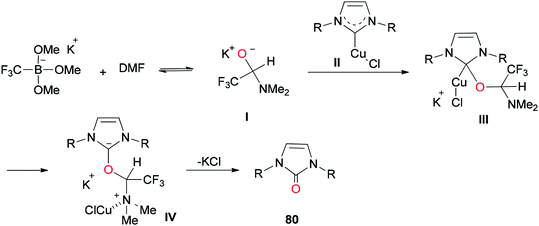
In 2013, a unique and efficient method was reported by Chen and co-workers for the formation of complicated imidazolinones 80 from carbene complexes 79 via an oxygen-atom insertion reaction of NHC-copper complexes using DMF as the source of oxygen (Scheme 36).122
A mechanistic scheme for the formation of complicated imidazolinones 80 is presented in Scheme 37. The negative oxygen ion intermediate I generated by reaction of the trifluoromethyl borate salt with DMF is transformed to less stable complex III through oxygen-atom insertion of NHC–copper complex II. Next, the copper chloride is dissociated and the carbanionic intermediate IV is produced in a similar path to Zhou's mechanism.123,124 At the late stage, IV undergoes a C–N bond cleavage, leading to the desired product 80.
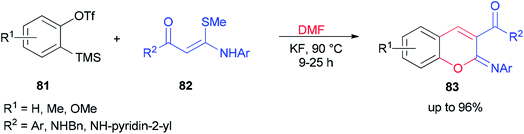
After a couple of years, Li and co-workers synthesized 2-aryliminochromenes 83 by a transition-metal-free reaction between arynes, N,S-keteneacetals 82 and dimethylformamide, where dimethylformamide acts as a source of oxygen to form chromeneskeleton 83 (Scheme 38).125
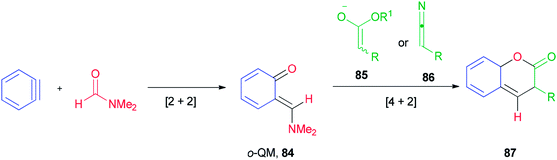
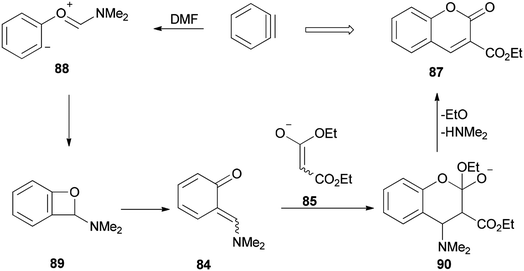
Ortho-Quinone methide 84 produced by [2 + 2] cycloaddition of aryne with DMF was efficaciously coupled with ester enolates or ketenimine anions under a [4 + 2] cycloaddition to provide direct access to various coumarins 87, which included integral and key parts of active biological compounds and pharmaceuticals (Scheme 39).126
The mechanism of this reaction is depicted in Scheme 40 and begins with a nucleophilic attack of oxygen of DMF to benzyne to afford the resulting zwitterion (88)127 which is subjected to intramolecular cyclization to furnish benzoxete 89. Then, an ortho-quinone methide 84 is obtained from 89 by electrocyclic ring-opening [4 + 2]. Cycloaddition of 84 with an enolate of 85, and then by elimination of a dimethylamine and ethoxide, gives 87.
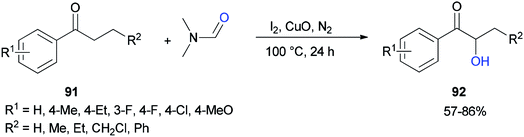
Liu and co-workers in 2017 used DMF as a source of oxygen for the production of α-hydroxy arones 92. Treatment of propiophenones 91 with DMF via α-hydroxylation reaction in the presence of iodine, copper oxide, and N2 afforded the corresponding α-hydroxylated products 92 (Scheme 41).128 The results demonstrated that the electronic effects of the substituents on the phenyl ring played a key role in this reaction. Among different copper species, CuO was found to be the most efficient oxidant.
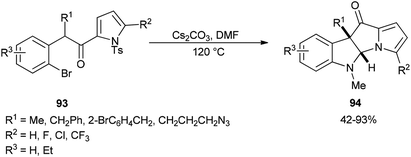
The formation of dihydropyrrolizino[3,2-b]indol-10-ones 94 was accomplished via a Cs2CO3-promoted cascade reaction of N-tosyl-2-(2-bromophenylacetyl)pyrroles 93 with DMF in absence of any exogenous transition metal catalyst or ligand (Scheme 42).129
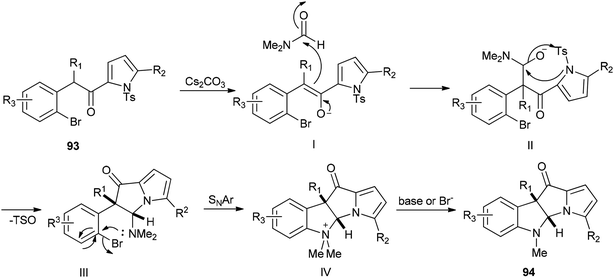
A plausible mechanism for this transformation is suggested through nucleophilic attack of DMF with enolate and pyrrolyl nitrogen followed by formation of C–N bond (Scheme 43).
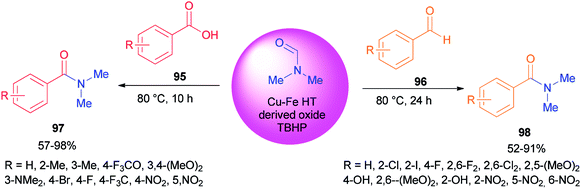
Recently, carboxylic acids 95 and substituted benzaldehydes 96 were amidated with DMF as a source of NMe2 in the presence of TBHP as an oxidant and Cu–Fe hydrotalcite-derived (HT-derived) as a catalyst by Priya and co-workers (Scheme 44).130 Cu–Fe HT-derived oxide which is highly active, is prepared from hydrotalcite-like materials formed by co-precipitation of Cu and Fe followed by calcination.
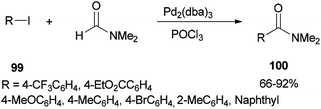
In 2002, Nozaki and co-workers presented a novel and brief strategy to synthesize arylcarboxamides 100 from aryl halides 99 and N,N-dimethylformamide both as an amide source in one step and as a solvent without the need for using carbon monoxide 100 (Scheme 45).131
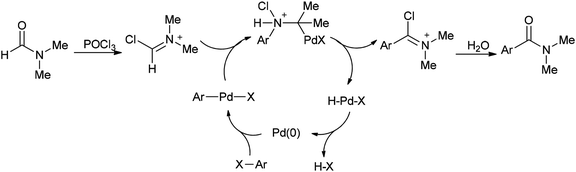
A mechanism for the synthesis of arylcarboxamides 100 has been proposed. Initially, Heck-type addition of aryl halide to C–N double bond in iminium species [Me2N+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl][Cl2P(
CHCl][Cl2P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O−] occurs which is followed by β-hydride elimination. It also is likely this reaction take place through oxidative addition of aryl halide to Pd(0) under reaction conditions to yield the arylpalladium halide (Scheme 46).132
O)O−] occurs which is followed by β-hydride elimination. It also is likely this reaction take place through oxidative addition of aryl halide to Pd(0) under reaction conditions to yield the arylpalladium halide (Scheme 46).132
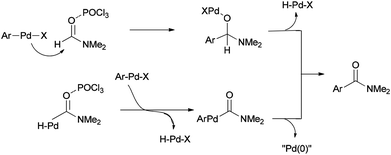
Two other proposed mechanisms are shown in Scheme 47. In the second mechanism, an amide product was produced through nucleophilic addition of arylpalladium halide to carbonyl activated with POCl3 (a Lewis acid) followed by β-hydride elimination. In the third mechanism, the reaction can be performed through oxidative addition of C–H bond of N,N-dimethylformamide and then by an exchange process and reductive elimination. The insertion of Ar–X to Ar-Pd-CONMe2, instead of the exchange furnished Pd(IV) may be an alternative and key precursor for the reductive elimination of product. It is important to note that in these three proposed mechanisms no base was used although in conventional examples, presence of a base for the regeneration of Pd(0) species from H–Pd–X is required.
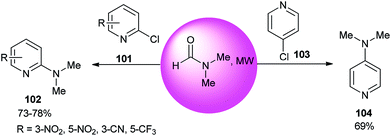
The reaction of 2- or 4-chloropyridine with DMF as an amine source and solvent under refluxing conditions afforded aminopyridines (Scheme 48).133
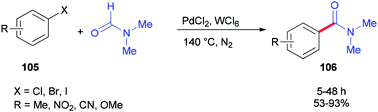
In 2014, Iranpoor and co-workers used WCl6/DMF as a Vilsmeier iminium type intermediate and a reducing system for generation of Pd(0) in aminocarbonylation of aryl halides 105. In this reaction, the corresponding N,N-dimethyl amides 106 were synthesized via treatment of aryl halides 105 (iodides, bromides, as well as chlorides) with N,N-dimethylformamide, using PdCl2 as a pre-catalyst without any phosphorous ligand under standard conditions (Scheme 49).134 Among metal halides, tungsten hexachloride (WCl6) was the most efficient. Reasonably, the Pd(II) was easily reduced to Pd(0) using WCl6/DMF in comparison with other metal halides (MoCl5, ZrOCl2, ZrCl4, TiCl4, or FeCl3) in DMF.
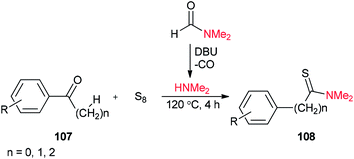
Thioamides 108 were produced in 2015 by Liu and co-workers from compounds 107, octasulfur and dimethylamine which was prepared in situ from DMF through a base-induced cleavage (Scheme 50).135
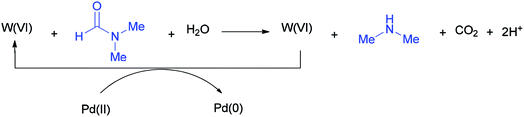
According to literature results, DMF acts as a reducing agent for the reduction of Ag(I) to Ag(0). On this basis, DMF can act as a reducing agent for the transformation of W(VI) to W(IV) which in turn acts as a reducing agent for the conversion of Pd(II) to Pd(0) (Scheme 51).136
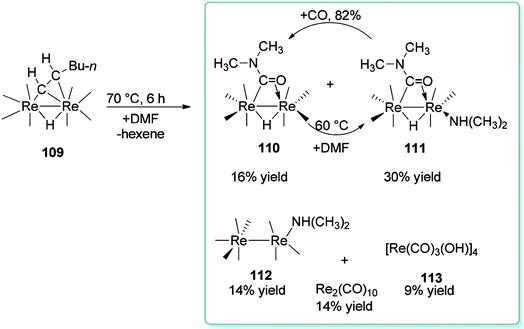
For the first time, Adams and Dhull reported the binuclear oxidation addition of a formyl C–H bond of DMF to produce a η2-bridging formamido (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNMe2) ligand with the C atom bonded to one rhenium (Re) atom and the O atom bonded to the other rhenium atom (Scheme 52).137 Elimination of hexane under heat in the presence of DMF led to three novel dirhenium products 110–112. Generation of small amounts of [Re(CO)3(OH)]4 113, HRe(CO)5, and Re2(CO)10 were the result of reactions of 109 and 110 with traces of H2O in the DMF reagent.138
CNMe2) ligand with the C atom bonded to one rhenium (Re) atom and the O atom bonded to the other rhenium atom (Scheme 52).137 Elimination of hexane under heat in the presence of DMF led to three novel dirhenium products 110–112. Generation of small amounts of [Re(CO)3(OH)]4 113, HRe(CO)5, and Re2(CO)10 were the result of reactions of 109 and 110 with traces of H2O in the DMF reagent.138
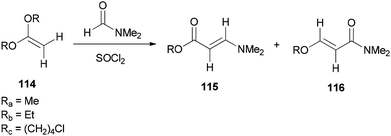
Bates's group presented a one-step method for the generation of alkyl (E)-3-(dimethylamino)acrylates 115 by reaction of ketene acetals 114 with DMF (Scheme 53).139
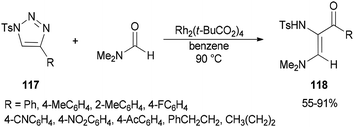
Incorporation of all the atoms of a DMF molecule into the product was achieved by a formal stereoselective 1,2-insertion of rhodium(II) azavinyl carbenes, produced in situ from N-sulfonylated 1,2,3-triazoles 117 using Rh2(t-BuCO2)4, into the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond of DMF to provide cis-diamino enones 118 (Scheme 54).140
O double bond of DMF to provide cis-diamino enones 118 (Scheme 54).140
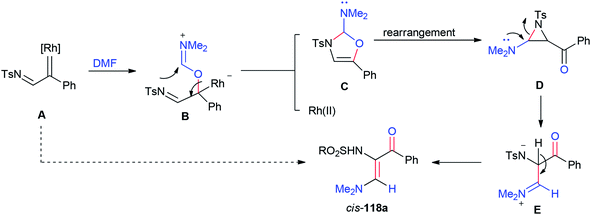
A plausible mechanism for the formation of 118a is illustrated in Scheme 55. Rhodium–iminium zwitterionic B prepared by stereoselective 1,2-insertion of A into the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of DMF resulted in the formation of 2-amino-4-oxazoline C as a product. The latter is rearranged to give aziridine D. At last, the synthesis of stable vinylogous enamino ketone 118a is completed by conversion of intermediate D by ring-opening and deprotonation.
O bond of DMF resulted in the formation of 2-amino-4-oxazoline C as a product. The latter is rearranged to give aziridine D. At last, the synthesis of stable vinylogous enamino ketone 118a is completed by conversion of intermediate D by ring-opening and deprotonation.
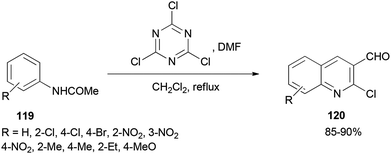
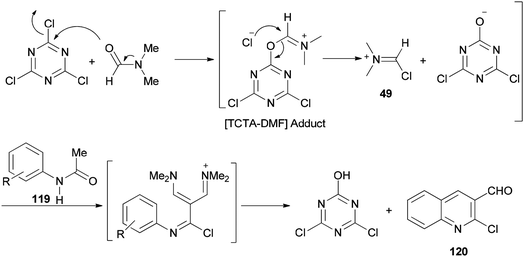
2-Chloroquinoline-3-carbaldehydes 120 were synthesized by Rajanna and co-workers through cyclization of acetanilides 119 with 2,4,6-trichloro-1,3,5-triazine/N,N-dimethylformamide (TCTA/DMF) adduct as a Vilsmeier–Haack type reagent under conventional and ultrasonically assisted reaction conditions (Scheme 56).141 The reaction time under sonication is reduced in comparison to when reactions are performed under conventional reaction conditions.
The mechanism starts with preparing [TCTA–DMF] adduct which is converted to (chloromethylene)dimethyliminium. The reaction of the cationic intermediate with acetanilide 119 furnished the 2-chloroquinoline-3-carbaldehydes 120 (Scheme 57).
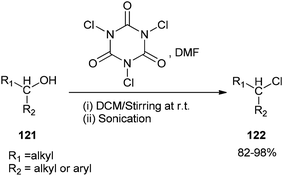
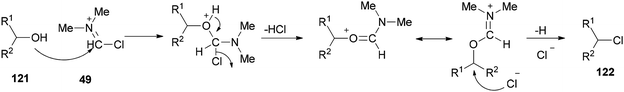
As a mild method, the smooth conversion of alcohols 121 to their corresponding alkyl chlorides 122 were accomplished and reported by Venkanna and co-workers in 2015 in the presence of TCCA/DMF in high yields with a relatively short reaction time (Scheme 58).142
As shown in Scheme 59, by addition of alcohol 121 to Vilsmeier–Haack (VH) complex 49, an interaction occurs between the hydroxyl group of the alcohol and choloromethyleniminium species (cationic species) which is followed by nucleophilic attack of chloride ion to generate the desired chloride 122. On the basis of observed stereochemical results, the reaction probably occurs through SN2 reaction mechanism.
For years, the Vilsmeier reaction has been used to achieve a key transformation such as formylation.143–146 There are reports of annulation of aliphatic substances including acyclic ketones,147–151 α,β-epoxy ketones,152 cyclohexenones,148,153 and etc.154–158 into their aromatic compounds upon treatment with POCl3/DMF as the Vilsmeier reagent. Generally, phosgene (COCl2) and phosphorus oxychloride (POCl3) are toxic reagents which are employed in traditional Vilsmeier–Haack reactions.159
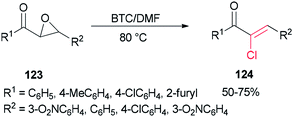
Therefore, BTC/DMF (bis(trichloromethyl) carbonate/N,N-dimethylformamide) adduct was used in 2007 as a Vilsmeier reagent for the effective synthesis of (Z)-2-chloro-1,3-diarylpropen-1-ones 124 from 2,3-epoxy-l,3-diarylpropan-1-ones 123, which were readily obtained by oxidation of chalcones using hydrogen peroxide as an oxidant (Scheme 60).160 The availability of starting materials and simplicity of manipulation make the present protocol a complement for an attractive synthetic method for academic research and potential applications.
The mechanism of the formation of (Z)-2-chloro-1,3-diarylpropen-1-ones 124 could be explained by an initial reaction of BTC with DMF to generate halomethylene iminium salts 49 followed by coordination with 2,3-epoxy-l,3-diarylpropan-1-ones 123 to furnish intermediates oxiranium A, as shown in Scheme 61. Next, intermediates B produced by attack of previously released halide anion from the Vilsmeier reagent on the benzyl position of A led to obtain dichloride D. At the end, one molecule of HCl from compound D is eliminated to yield the desired products 124.
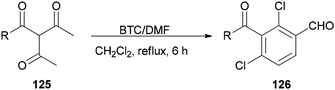
Su and co-workers presented a convenient method for the production of a series of substituted aromatic compounds 126 using BTC/DMF as Vilsmeier reagent in refluxing dichloromethane (Scheme 62).161 When reaction temperature was increased, decreasing yield and reaction complexity was observed. As an example, the reaction did not occur in DMF. Hence, the best results were obtained by using BTC/DMF in refluxing CH2Cl2 for 6 h.
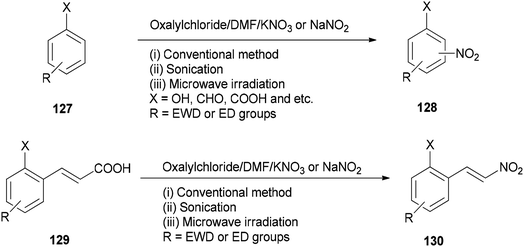
As a methodology, generation of the corresponding nitro derivatives 128 and 130 was reported and achieved via nitration of aromatic compounds 127 and cinnamic acids 129 with (COCl)2/DMF using KNO3 or NaNO2 under microwave and ultrasonic conditions, respectively (Scheme 63).162 In this transformation, the use of microwave and ultrasonic irradiation lead to enhancing the yield of products and reducing reaction time. The main advantages of this present methodology are: (i) work-up procedure is simple; (ii) the products are prepared from readily available and economically cheap reagents; (iii) yields are excellent; and (iv) the reaction time is short.
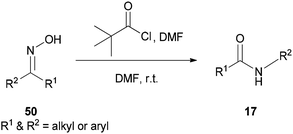
A new protocol for mild, efficient, and general conversion of ketoximes 50 to their corresponding amides 17 was developed by Narahari and co-workers in 2011 through a Beckmann rearrangement using an inexpensive and nontoxic reagent (pivaloyl chloride/DMF complex) in DMF as the solvent (Scheme 64).163 Classical Beckmann rearrangements were generally performed in the presence of strongly acidic and dehydrating media at high temperatures97,164–166 while formation of a pivaloyl chloride/DMF complex in this method was achieved at room temperature without the use of acid.
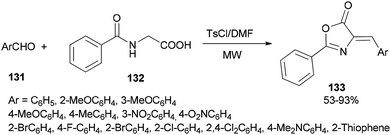
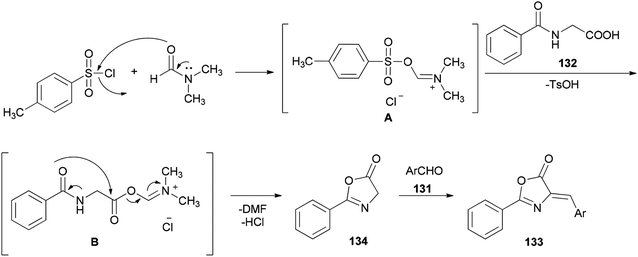
A simple and efficient protocol was designed and reported by Moghanian's group in 2012 for synthesis of azlactone derivatives 133 through a condensation reaction of aromatic aldehydes 131 with hippuric acid 132 using a TsCl and DMF system as condensing agent under microwave irradiation (Scheme 65).167 In order to obtain appropriate conditions for this reaction, different bases, such as DMF, pyridine, sodium acetate, and triethylamine were examined in the presence of TsCl under microwave irradiation. Among the bases, DMF gave the best result.
Scheme 66 shows a possible mechanism for synthesis of azlactone derivatives 133. The first step involves the formation of the Vilsmeier adduct B from the treatment of hippuric acid 132 with TsCl and DMF. Then, the intermolecular cyclization of intermediate B, produced the azlactone 134 based on good evidence via Erlenmeyer synthesis.168,169 Finally, aldol condensation of 134 with carbonyl compounds 131 afforded the desired compounds 133.
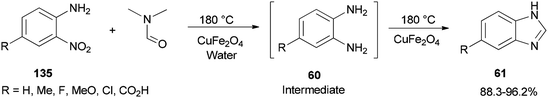
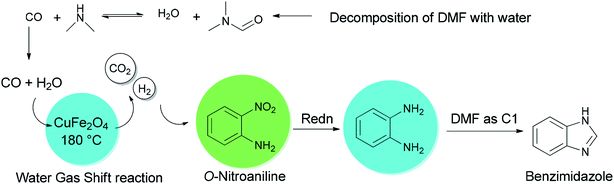
Quite recently, Yadav and Rasal presented an economical synthetic protocol for preparation of benzimidazoles 61 from o-nitroanilines 135 using N,N-dimethylformamide as the source of C1 and magnetically separable CuFe2O4 as a cheap and recyclable catalyst in a one pot reaction with 100% conversion in 12 h with 97.5% selectivity (Scheme 67).170
A mechanistic scheme for a one-pot synthesis of benzimidazoles 61 is shown in Scheme 68. The reaction commences with decomposition of DMF to CO and NMe2. Next, H2 and CO2 are formed from the in situ generated CO under water gas shift reaction (WGSR) conditions using CuFe2O4. The NO2 group in o-nitroaniline is converted to NH2 group in o-phenylenediamine via reduction using H2 and CuFe2O4. The resulting compound is cyclized in the presence of DMF as a C1 source to afford benzimidazole.
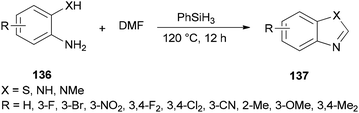
Liu and co-workers reported the synthesis of heterocycles 137 from 2-phenylenediamines 136 and DMF using PhSiH3 as the only promoter without the need for any other catalysts or additives under metal-free conditions (Scheme 69).171
3. DMF as a catalyst
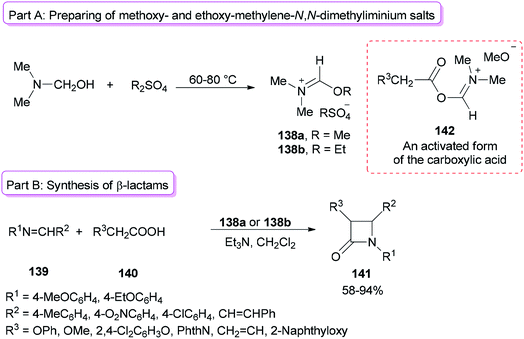
Alkoxymethylene-N,N-dimethyliminium salts were exploited in the catalysis of the Beckmann rearrangement,172 preparing 3-acylated indolizines,173 and synthesis of benzylidene acetals of mono- and disaccharides.174Formation of 2-azetidinones 141 via a unique reaction between Schiff bases, substituted acetic acids, and alkoxymethylene-N,N-dimethyliminium salts was reported by Jarrahpour and co-workers in 2009 (Scheme 70).175 The remarkable advantages of this method include low cost, mild reaction conditions, and non-use chlorinating agents as well as easy purification of the products. Treatment of DMF with Me2SO4 or Et2SO4 afforded methoxy- or ethoxy-methylene-N,N-dimethyliminium salts 138a,b (Scheme 70, part A). It should be noted that Me2SO4 or DMF alone is inactive. The obtained salt 138a from DMF/Me2SO4 showed better activity than derived salt 138b from DMF/Et2SO4 (Scheme 70, part B). The best results were gained when DMF and Me2SO4 were used at room temperature. As a proposal, the reaction just progressed through generation of an activated form of the carboxylic acid 142 which in the following, the corresponding ketene is prepared under deprotonation and loss of DMF from 142.
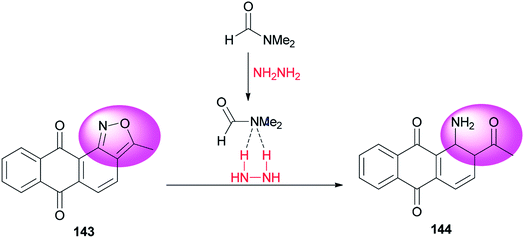
A clean, facile and highly efficient pathway for synthesis of 1-amino-2-acetylanthraquinone 144 (95.0% selectivity) by DMF-promoted reductive ring-cleavage of an isoxazole motif-containing 3-methyl-anthra-[1,2-c]-isoxazol-6,11-dion 143 was presented by Zhao and co-workers (Scheme 71).10 N,N-Dimethylformamide in this transformation plays dual roles as catalyst and reaction media. The catalytic effect of DMF occurs from formation of a H2N–NH2/DMF complex by hydrogen bonding.
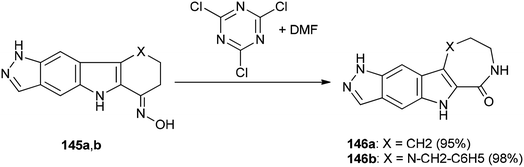
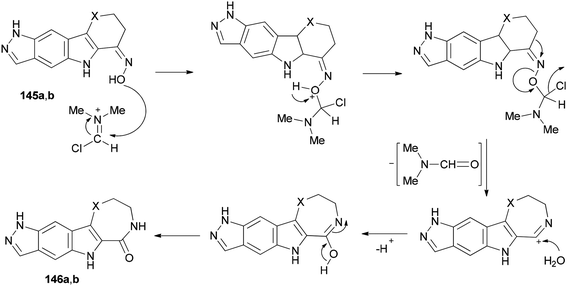
Singh and co-workers in 2013 achieved and reported a very mild protocol for regioselective synthesis of the corresponding azepine 146a and 1,4-diazepine 146b through a Beckmann rearrangement of ketoximes of pyrazolo annulated oxocarbazole 145a and oxoazacarbazole 145b with an organocatalyst derived from DMF and TCTA (Scheme 72).12
Scheme 73 shows a rational mechanistic pathway for formation of 146a,b from 145a,b. The reaction was carried out through preparation of a complex derived from TCT as a cheap reagent and DMF, that allowed the Beckmann rearrangement to be accomplished with 145a,b to yield 146a,b, respectively.
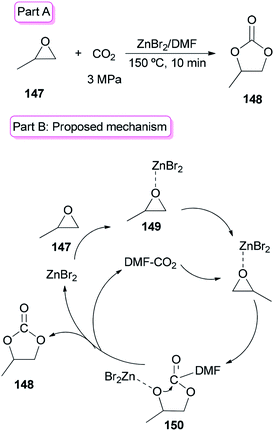
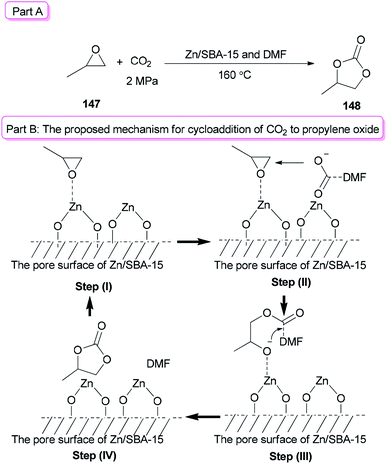
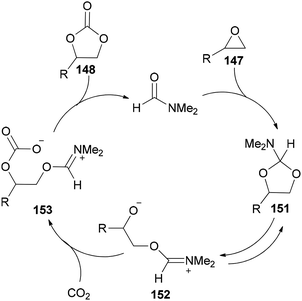
Sun and co-workers prepared the cyclic carbonate 148 by cycloaddition of propylene oxide 147 with CO2 catalyzed by ZnBr2/DMF in 2005 (Scheme 74, part A).42 ZnBr2 coupled with a small amount of DMF was converted to highly active species. Interestingly, DMF in a small amount interacted as a co-catalyst with CO2 in the gas phase while in a large amount, served as activator and solvent of CO2 in the liquid phase. Propylene carbonate 148 was produced in 56.5% yield with 100% selectivity using 3 MPa of CO2. The ZnBr2/DMF catalyst reported herein compared to the other reported catalysts, is facile available and cost-effective. This catalyst also showed high selectivity/activity, simultaneously. A probable catalytic cycle is proposed in Scheme 74, part B. The reaction began through coordination of PO with ZnBr2 to provide the adduct of zinc–epoxide complex 149, which simply gave a propylene oxide open ring; next, the oxy anion of complex 150 was generated by attack of the carboxylate anion (which is formed by CO2 activated with DMF) to the less hindered carbon atom of PO; at the late stage, the propylene carbonate 148 is produced by attack of oxy anion to DMF bonded CO2 and breaking of the bond Zn–O concurrently, followed by cyclic elimination, in an intermolecular fashion.
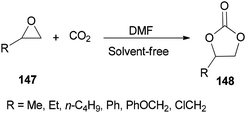
DMF is a simple, small molecule, and cost-effective organic catalyst which was used for the synthesis of cyclic carbonates 148 via coupling reaction of epoxides 147 with CO2 under solvent-free conditions by Jiang and Hua in 2006 (Scheme 75).43
Scheme 76 displays a probable mechanism for this transformation. The conversion of 151 to imine salt 152 and then its reaction with CO2 afforded the intermediate 153. Finally, the formation of C–O bond provides cyclic carbonate 148 and regenerates DMF.
 | ||
| Scheme 76 A probable mechanism for cycloaddition of epoxides 147 with CO2 using DMF under solvent-free conditions. | ||
In recent years, metal oxides as inexpensive and simple heterogeneous catalysts have been developed,176 while their big draw backs were noticeable.176–180 For instance: (1) the use of much higher catalyst/substrate ratios to metal oxides was frequently required; (2) usage of large amounts of a toxic solvent was frequently needed for metal oxides; and (3) they displayed a low activity/selectivity for cycloaddition of CO2. To solve problems of the metal oxides, they were loaded onto mesoporous SBA-15. In this method, the surface of metal oxides enhanced the reaction and the amount of metal oxides used decreased. In addition, a small amount of DMF was applied as a catalyst instead of a large amount of organic solvent. Zn/SBA-15 (0.15) as the catalyst formed via a one-pot synthetic method was used for cycloaddition reaction of CO2 to propylene oxide (PO) 147 in the presence of DMF to give propylene carbonate (PC) 148 in excellent yield (92.3%) (Scheme 77, part A).41 A probable mechanism for this reaction is proposed (Scheme 77, part B). In the first step, the Lewis acid center of zinc on the SBA-15 surface activated the propylene oxide. In the second step, the carboxylate anion, which was produced by coordination of nitrogen of DMF with CO2, attacked the less sterically hindered carbon atom of PO. As a result, the negative charge of the carboxylate anion was converted to the oxygen atom of PO. The propylene carbonate was generated by intramolecular nucleophilic attack of the oxygen anion on the carbon atom of CO2 bonding with DMF (step III) followed by ring-closure reaction (step IV).
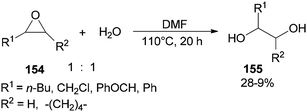
Production of 1,2-diols 155 was performed via hydrolysis of epoxides 154 with H2O (one equimolar) using DMF. This organocatalytic procedure offers remarkable advantages, such as low cost of the catalyst, mild reaction conditions, and easy separation of the product (Scheme 78).181
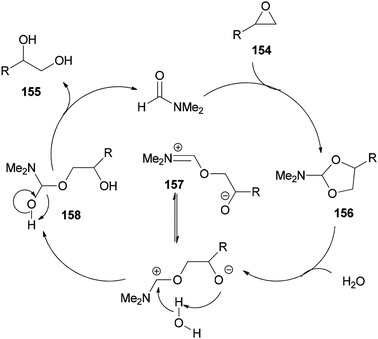
As shown in Scheme 79, epoxide 154 is treated with DMF to furnish N,N-dimethylformamide ethylene acetal derivative 156. The ring of 156 is opened in the presence of H2O to give the species 157. The vicinal diol 155 and regenerated DMF are obtained by C–O bond cleavage of intermediate 158 which is, in turn, produced from 157.
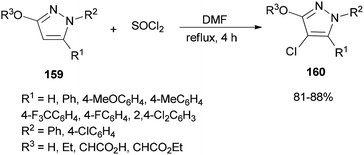
A simple, brief, and efficient method was developed by Zhu and co-workers in 2011 to produce 4-chloro-3-oxypyrazoles 160 by treatment of 3-oxypyrazoles 159 with SOCl2 using catalytic amounts of DMF (Scheme 80).36 The salient features for this monohalogenation method included high regioselectivity, good functionality tolerance, quick access to products, and experimental simplicity. In this reaction, the catalyst DMF plays a vital role both as an electron-withdrawing group and as a leaving group upon protonation.
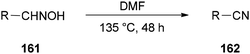
Nitriles 162 were obtained by Varvounis and co-workers in 2007 through dehydration of aldoximes 161 by employing DMF as catalyst and solvent (Scheme 81).182
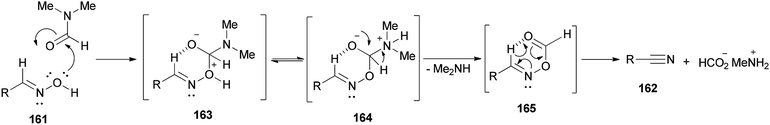
It is mechanistically suggested that the reaction occurs by initial attack of the hydroxyl group of the oxime 161 on the carbonyl group of dimethylformamide to yield the cyclic intermediate 163 (Scheme 82). It can lose the proton of hydroxyl to convert to the nitrogen atom of intermediate species 164. Next, the intramolecularly hydrogen bonded aldoxime formate as an intermediate 165 is formed by removal of dimethylamine from 164 which was transformed to the expected nitrile 162 by subsequent thermal elimination of formic acid.
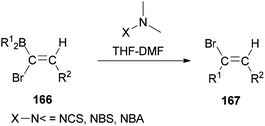
In 2000, and for the first time, a novel and general pathway to synthesize 1,2-disubstituted (E)-vinyl bromides 167 was performed by DMF-induced reaction of [(Z)-1-bromo-1-alkenyl]dialkylboranes 166 with a N-halogeno compound (Scheme 83).183 But, it is important to note that this reaction in the absence of N,N-dimethylmethanamide provided only a trace amount of desired product.
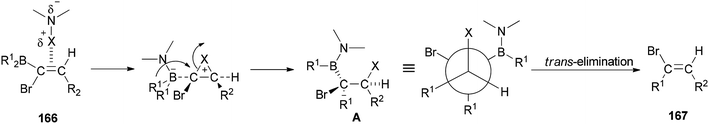
A mechanistic proposal for the synthesis of 167 is displayed in Scheme 84. It starts through electrophilic attack of the N-halogeno compound. It is supposed that the construction of a halonium ion across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is followed by 1,2-migration of an alkyl group from the dialkylboryl group to the α-carbon atom to give intermediate A. Next, the latter under trans-elimination produces the 2-disubstituted (E)-vinyl bromides 167.
C bond is followed by 1,2-migration of an alkyl group from the dialkylboryl group to the α-carbon atom to give intermediate A. Next, the latter under trans-elimination produces the 2-disubstituted (E)-vinyl bromides 167.
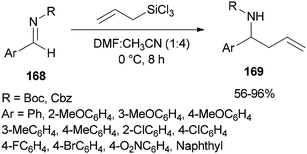
Syntheses of N-Boc and N-Cbz homoallylic amines 169 was achieved and presented in 2008 by Wu and Sun through facile allylation of various N-Boc and N-Cbz imines 168 with allyltrichorosilanes as an allylating agent using N,N-dimethylformamide (dual roles, as an activator and solvent) (Scheme 85).184 Interestingly, in the absence of DMF, the reaction was not achieved.
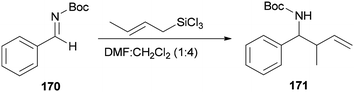
Wu and Sun also prepared the desired product 171 as a syn/anti mixture by crotylation of 170 with (E)-crotyltrichlorosilanes under conditions of DMF/CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (Scheme 86). The performed reaction at −20 °C and 0 °C yielded product 171 in 78% yield (syn/anti (4
4) (Scheme 86). The performed reaction at −20 °C and 0 °C yielded product 171 in 78% yield (syn/anti (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6)) and 37% (syn/anti (5
6)) and 37% (syn/anti (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6)), respectively.184
6)), respectively.184
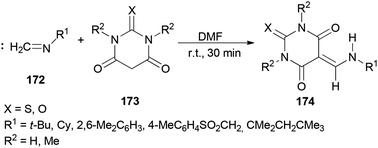
In 2010, Teimouri and Tayyebi synthesized 5-[(alkyl or arylamino)methylene]barbituric acids 174 by DMF promoted condensation reactions of alkylisocyanides (or arylisocyanides) 172 with barbituric acid derivatives 173 (Scheme 87).13 Compared with the other methods,185–191 this methodology has six incomparable advantages: (1) the reaction is simple; (2 and 3) the reaction performs at room temperature and without the use of any other catalyst; (4) yields are good to excellent; (5) products are produced within a short time; and (6) the products are sufficiently pure.
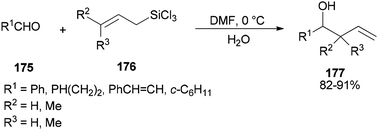
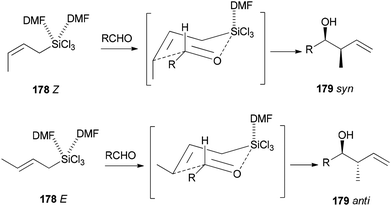
Synthesis of syn- and anti-homoallylic alcohols 177 was achieved by smooth treatment of aldehydes 175 with (Z)- or (E)-allyltrichlorosilanes 176 in DMF without any other catalyst, respectively (Scheme 88).44
Novel C–C bonds were formed only at γ-positions of allyltrlchlorosilanes. As for the transition state, a six-membered molecule is suggested for the allylation of aldehydes (Scheme 89).192–194 Among several solvents, DMF is the only solvent that was able to ensue this reaction. The 29Si NMR spectra of 1Z showed that N,N-dimethylformamide coordinated to the Si atom of 1Z to yield the resulting five or six coordinated organosilicate. The organosilicate as a hypervalent molecule has enough Lewis acidity and nucleophilicity, to enable the reaction to proceed smoothly and stereoselectively.
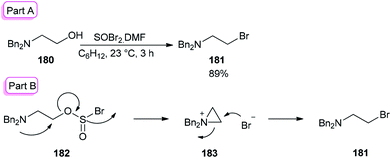
The smooth conversion of β-aminoalcohols to β-amino bromides was accomplished in the presence of DMF and thionyl bromide (SOBr2) by Jung and co-workers in 2000.195 This method is also applicable for the conversion of both primary and secondary alcohols to their corresponding bromides in high yields.
As an example, the reaction of N,N-dibenzylamino ethanol 180 with SOBr2 and DMF afforded the expected bromide 181 in excellent (89%) yield and purity (Scheme 90). DMF as a catalyst accelerates the reaction considerably via a Vilsmeier–Haack type SOBr2–DMF complex. In order to gain appropriate conditions for this method, Jung's group used different polar and nonpolar solvents, such as cyclohexane, hexanes, petroleum ether, dichloromethane, etc. Among the solvents, cyclohexane was shown to give the best result.
An AlCl3–DMF complex was employed as a catalyst in a Friedel–Crafts reaction.196–198 In 2008, Poupaert and co-workers presented Friedel–Crafts acylation of aromatic compounds, such as anisole 185, 3-methyl-2(3H)-benzoxazolone, and 3-methyl-2(3H)-benzothiazolone 187 for synthesis of their aryl ketones using I2/DMF as a catalyst. The reaction is carried out under conditions including minimal energy-consumption and minimal wastes. At the end of the reaction, catalyst and wastes are innocuous for the environment (Scheme 91).46 The interaction of iodine with the oxygen atom of DMF formed I2/DMF as a stable complex. This catalyst exhibits a unique behavior in the Friedel–Crafts acylation of aromatic compounds bearing electron-donating groups.
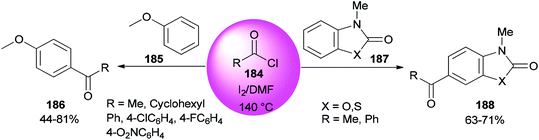 | ||
| Scheme 91 Acylation of anisole 185 and 3-methyl-2(3H)-benzoxazolone and 3-methyl-2(3H)benzothiazolone substrates 187. | ||
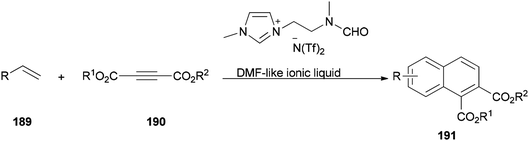
The DMF-based ionic liquid was applied as a nucleophilic reagent and as a solvent for preparing a variety of 1,2 disubstituted-3,4-dihydronaphthalenes 191 by cycloaddition of various vinylarenes 189 with electron-deficient alkynes 190 by Hullio and his group, recently (Scheme 92).199
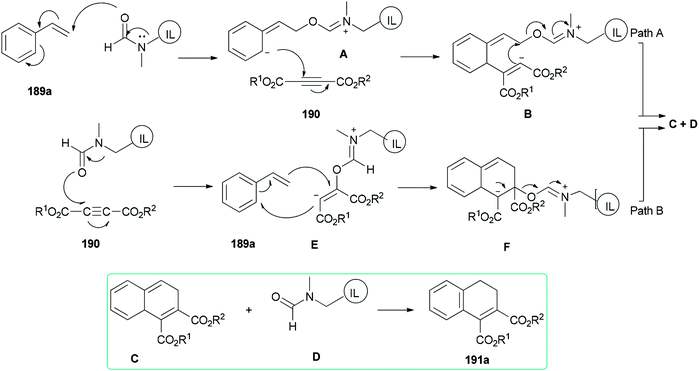
Two possible mechanisms are depicted in Scheme 93: (a) nucleophilic attack of the oxygen of DMF-based ionic liquid to the β-carbon of vinylic carbon of styrene 189a to afford the zwitter ionic intermediate A. Next, another zwitter ion intermediate B, which is constructed by nucleophilic attack of the anionic carbon to the acetylenic carbon of acetylene dicarboxylate 190, converted to product C and regenerated ionic liquid D under the indicated bonding arrangements. The corresponding compound A undergoes aromatization to afford the expected product 191a (Scheme 93, Path A). (b) Nucleophilic attack of the oxygen of DMF-based ionic liquid D to the acetylenic carbon of acetylene dicarboxylate 190 to form a highly charged reactive intermediate E. The anionic carbon of E attacks the beta carbon of 189a with the production of another intermediate F that undergoes bond shuffling to obtain C with regeneration of a DMF-based ionic liquid. Finally, the expected product 191a is provided by restoration of aromaticity (Scheme 93, Path B).
4. DMF as a stabilizer
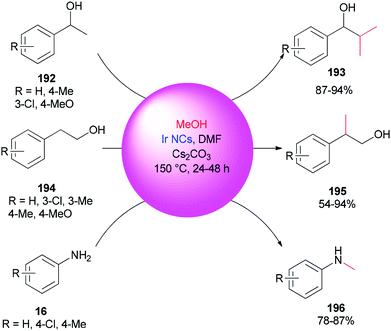
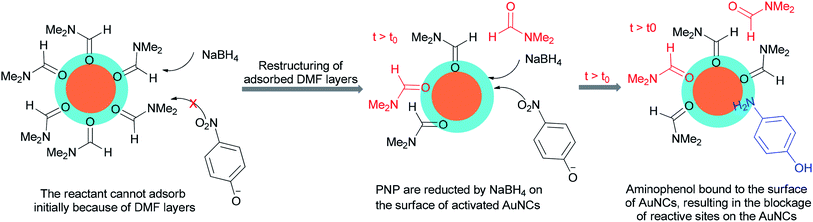
An alternative and efficient methodology for a solution preparation of DMF-stabilized metal nanoclusters (NCs < 2 nm) and nanoparticles (NPs > 2 nm) of Pt, Au, Cu, and Pd as surfactant-free stable M NCs has been reported.47,48,50,51 In this methodology, metal nanoparticles are synthesized using DMF as a solvent, reductant, and stabilizer. The protocol has no need for any external additives. Quite recently, DMF-stabilized Ir nanoclusters were applied as catalysts for methylation of alcohols 192 and 194 and anilines 16 using MeOH as the C1 source by Obora and co-workers (Scheme 94).53 These catalysts, which were synthesized in particle sizes of 1–1.5 nm in one step, exhibited an effective catalytic activity in the β-methylation of various primary and secondary alcohols in the presence of MeOH as the source of C1.Kawasaki and co-workers in 2012 investigated the catalytic effect of DMF-stabilized AuNCs in the reduction of 4-nitrophenol (PNP) to 4-aminophenol by sodium borohydride.11 A mechanism for AuNC-catalyzed reduction of 4-nitrophenol is suggested as shown in Fig. 2.11 Kawasaki proposed that the beginning of the reaction included two steps: initially, the layers of DMF were regenerated, and molecules of DMF partly desorbed from the AuNCs surface. In the second step, PNP penetrated onto the active surface of Au through diffusion. These processes afforded an induction time (t0), after which the nanoclusters as catalysts facilitated transfer of electrons from BH4− to 4-nitrophenol.
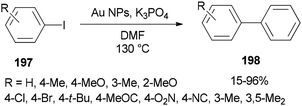
In 2014, Lang and co-workers prepared DMF-stabilized gold nanoparticles by a DMF reduction protocol. By changing reaction time and temperature, the size of the gold nanoparticles could be controlled. Among different sizes of Au NPs, 2.5 nm indicated the highest catalytic activity for Ullmann homocoupling reaction of aryl iodides 197. Apart from DMF, this catalytic coupling reaction does not require any other organic ligand; DMF serves as a reductant, stabilizer, and solvent in the synthesis of metal NP (Scheme 95).54
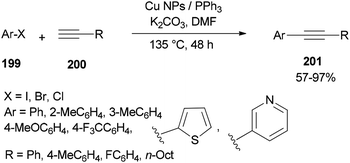
The DMF-stabilized Cu NPs catalyst which was produced exhibited a high catalytic activity in a Sonogashira cross-coupling reaction (Scheme 96).52 This synthetic catalyst can also be used in reactions of a wide range of substrates, such as aryl halides 199 and alkynes 200 bearing electron releasing groups (ERG) and electron withdrawing groups (EWG), as well as heterocyclic and sterically hindered substrates. Obora's group found that the reactions could be successfully performed with low loadings of the catalyst.
The Migitae–Kosugie–Stille cross-coupling reaction is based on organostannanes catalyzed by Pd complexes.200–204 This reaction was applied in preparing biaryl compounds which are key and vital intermediates in the syntheses of functionalized polymers, pharmaceuticals,205–208 and natural products.209,210
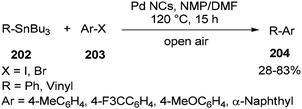
In 2013, synthesis of biaryls and vinylarenes was reported through the Migitae–Kosugie–Stille cross-coupling reaction using N,N-dimethylformamide-stabilized palladium nanoclusters.55 The Migitae–Kosugie–Stille reaction between arylstannane (vinylstannane) 202 with aryl halide 203 in the presence of DMF-stabilized Pd NCs as an efficient and highly active catalyst in the mixture of NMP/DMF (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) afforded biaryl (vinylarene) 204 (Scheme 97). It was reported that the palladium-catalyzed Migita–Kosugi–Stille reaction is promoted by addition of copper iodide as an additive. Product 204 was provided in excellent yield by addition of CuI.
1) afforded biaryl (vinylarene) 204 (Scheme 97). It was reported that the palladium-catalyzed Migita–Kosugi–Stille reaction is promoted by addition of copper iodide as an additive. Product 204 was provided in excellent yield by addition of CuI.
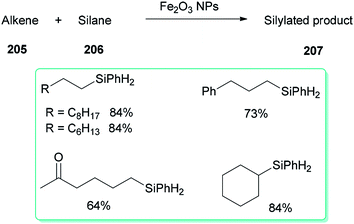
A DMF reduction method was used for production of a monodispersed DMF-stabilized Fe2O3 NPs (2–5 nm) catalyst by employing Fe(acac)3 as a precursor under open air conditions. This novel catalyst displayed efficient catalytic activity for hydrosilylation of an alkene 205 with hydrosilane 206, resulting in versatile silylation products 207, without the need for any additives (Scheme 98).57
5. Conclusion
This review underscores recent progress in exploiting DMF as a privileged chemical, thanks to its structure. It is a multipurpose compound besides being an effective polar solvent. It acts as a reagent in various reactions such as formylation, amination, aminocarbonylation, amidation, and nitrilation, as well as reactions with arynes. Particularly, employment of DMF as a precursor in nitrilation and formylation reactions have seen important developments. It is noteworthy that in some reactions other amides can be used as precursors as well. With the concern in green and sustainable chemistry, it can be anticipated that application of DMF as a precursor will continue to develop in organic synthesis. Moreover, the development of new catalytic systems with increased reactivity will have important consequences for the practical utilization of DMF as a catalyst. Mechanistic studies in both reported reactions and future developments of novel reactions were conducted. Moreover, applications of DMF as a stabilizer were also discussed. In summary, in this review the main achievements on usage of DMF as a reagent, catalyst, and stabilizer have been summarized and discussed. We hope that it is sufficiently impressive and thorough that it will attract the attention of synthetic organic chemists and will initiate further developments in the applications of DMF beyond being just a polar solvent because it can be so much more with a little innovation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the Research Council of Alzahra University. MMH is also appreciate the financial support granted by Iran National Science Foundation (INSF) under the given individual research chair.References
- M. Sittig, Handbook of Toxic and Hazardous Chemicals and Carcinogens, Noyes Publications, Park Ridge, NJ, 1985 Search PubMed.
- J. Amoore and E. Hautala, J. Appl. Toxicol., 1983, 3, 272–290 CrossRef PubMed.
- A. Parker, Adv. Phys. Org. Chem., 1967, 5, 173–235 CrossRef.
- M. A. Meier, M. Filali, J.-F. Gohy and U. S. Schubert, J. Mater. Chem., 2006, 16, 3001–3006 RSC.
- J. Sharma, S. Mahima, B. A. Kakade, R. Pasricha, A. Mandale and K. Vijayamohanan, J. Phys. Chem. B, 2004, 108, 13280–13286 CrossRef.
- K. Osakada, A. Taniguchi, E. Kubota, S. Dev, K. Tanaka, K. Kubota and T. Yamamoto, Chem. Mater., 1992, 4, 562–570 CrossRef.
- H. Durmaz, A. Dag, O. Altintas, T. Erdogan, G. Hizal and U. Tunca, Macromolecules, 2007, 40, 191–198 CrossRef.
- P. Golas, N. Tsarevsky, B. Sumerlin and K. Matyjaszewski, Macromolecules, 2006, 39, 6451–6457 CrossRef.
- H. Do and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 1128–1129 CrossRef PubMed.
- Z. Zhao, R. Li and Y. Li, Sci. Lett. J., 2013, 2, 41 Search PubMed.
- H. Yamamoto, H. Yano, H. Kouchi, Y. Obora, R. Arakawa and H. Kawasaki, Nanoscale, 2012, 4, 4148–4154 RSC.
- R. Tyagi, N. Kaur, B. Singh and D. Kishore, Synth. Commun., 2013, 43, 16–25 CrossRef.
- M. B. Teimouri and A. Tayyebi, J. Chem. Res., 2010, 34, 140–144 CrossRef.
- S. Ahmad, D. Braddock, G. Cansell and S. Hermitage, Tetrahedron Lett., 2007, 48, 915–918 CrossRef.
- M. Semsarzadeh, S. Amiri and M. Azadeh, Bull. Mater. Sci., 2012, 35, 867–874 CrossRef.
- H. Bipp and H. Kieczka, Formamides, Wiley-VCH, Weinheim, 2000 Search PubMed.
- C. M. Hsu and S. Shivkumar, Macromol. Mater. Eng., 2004, 289, 334–340 CrossRef.
- L. Du, H. Xu, Y. Zhang and F. Zou, Fibers Polym., 2016, 17, 751–759 CrossRef.
- R. Kumar, D. Wadhwa and O. Prakash, Heterocycl. Commun., 2010, 16, 201–205 Search PubMed.
- A. Vilsmeier and A. Haack, Ber. Dtsch. Chem. Ges., 1927, 60, 119–122 CrossRef.
- W. Jackson, A. Sargeson, P. Tucker and A. Watson, J. Am. Chem. Soc., 1981, 103, 533–540 CrossRef.
- O. Meth-Cohn and B. Narine, Tetrahedron Lett., 1978, 19, 2045–2048 CrossRef.
- L. Bell, H. McGuire and G. Freeman, J. Heterocycl. Chem., 1983, 20, 41–44 CrossRef.
- M. P. Reddy and G. K. Rao, J. Org. Chem., 1981, 46, 5371–5373 CrossRef.
- A. R. Katritzky and C. M. Marson, J. Am. Chem. Soc., 1983, 105, 3279–3283 CrossRef.
- D. Adams, J. Dominguez and J. Perez, Tetrahedron Lett., 1983, 24, 517–518 CrossRef.
- O. Meth-Cohn and S. P. Stanforth, Compr. Org. Synth., 1991, 2, 777–794 Search PubMed.
- M. Venugopal and P. Perumal, Synth. Commun., 1991, 21, 515–519 CrossRef.
- K. Dinakaran and P. Perumal, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2000, 39, 135–136 Search PubMed.
- M. S. C. Pedras and M. Jha, J. Org. Chem., 2005, 70, 1828–1834 CrossRef PubMed.
- E. G. Mata and A. G. Suárez, Synth. Commun., 1997, 27, 1291–1300 CrossRef.
- S. Ahmed and R. Boruah, Tetrahedron Lett., 1996, 37, 8231–8232 CrossRef.
- J. Elguero and B. Shimizu, An. Quim., Ser. C, 1988, 84, 191–195 Search PubMed.
- J. Muzart, Tetrahedron, 2009, 65, 8313–8323 CrossRef.
- A. Dubey, A. Upadhyay and P. Kumar, Tetrahedron Lett., 2010, 51, 744–746 CrossRef.
- Y. Liu, G. He, K. Chen, Y. Jin, Y. Li and H. Zhu, Eur. J. Org. Chem., 2011, 2011, 5323–5330 CrossRef.
- A. Rai, V. K. Rai, A. K. Singh and L. D. S. Yadav, Eur. J. Org. Chem., 2011, 2011, 4302–4306 CrossRef.
- M. Gowda, S. Pande, R. Ramakrishna and K. Prabhu, Org. Biomol. Chem., 2011, 9, 5365–5368 RSC.
- J. Clayden, Organolithiums: Selectivity for Synthesis, Oxford University Press, Oxford, 2001 Search PubMed.
- M. Ansell, Preparation of Acyl Halides, John Wiley and Sons, London, 1972 Search PubMed.
- S. Zhong, L. Liang, M. Liu, B. Liu and J. Sun, J. CO2 Util., 2015, 9, 58–65 CrossRef.
- J. Sun, S.-I. Fujita, F. Zhao and M. Arai, Appl. Catal., A, 2005, 287, 221–226 CrossRef.
- J. L. Jiang and R. Hua, Synth. Commun., 2006, 36, 3141–3148 CrossRef.
- S. Kobayashi and K. Nishio, Tetrahedron Lett., 1993, 34, 3453–3456 CrossRef.
- A. S. Nagle, R. N. Salvatore, B.-D. Chong and K. W. Jung, Tetrahedron Lett., 2000, 41, 3011–3014 CrossRef.
- F. Guenadil, H. Aichaoui, D. Lambert, C. R. McCurdy and J. Poupaert, Lett. Org. Chem., 2008, 5, 665–668 CrossRef.
- X. Liu, C. Li, J. Xu, J. Lv, M. Zhu, Y. Guo, S. Cui, H. Liu, S. Wang and Y. Li, J. Phys. Chem. C, 2008, 112, 10778–10783 CrossRef.
- H. Kawasaki, H. Yamamoto, H. Fujimori, R. Arakawa, Y. Iwasaki and M. Inada, Langmuir, 2009, 26, 5926–5933 CrossRef PubMed.
- H. Kawasaki, H. Yamamoto, H. Fujimori, R. Arakawa, M. Inada and Y. Iwasaki, Chem. Commun., 2010, 46, 3759–3761 RSC.
- M. Hyotanishi, Y. Isomura, H. Yamamoto, H. Kawasaki and Y. Obora, Chem. Commun., 2011, 47, 5750–5752 RSC.
- Y. Isomura, T. Narushima, H. Kawasaki, T. Yonezawa and Y. Obora, Chem. Commun., 2012, 48, 3784–3786 RSC.
- H. Oka, K. Kitai, T. Suzuki and Y. Obora, RSC Adv., 2017, 7, 22869–22874 RSC.
- K. Oikawa, S. Itoh, H. Yano, H. Kawasaki and Y. Obora, Chem. Commun., 2017, 53, 1080–1083 RSC.
- W. Yao, W.-J. Gong, H.-X. Li, F.-L. Li, J. Gao and J.-P. Lang, Dalton Trans., 2014, 43, 15752–15759 RSC.
- H. Yano, Y. Nakajima and Y. Obora, J. Organomet. Chem., 2013, 745, 258–261 CrossRef.
- I. Pastoriza-Santos and L. M. Liz-Marzán, Pure Appl. Chem., 2000, 72, 83–90 Search PubMed.
- R. Azuma, S. Nakamichi, J. Kimura, H. Yano, H. Kawasaki, T. Suzuki, R. Kondo, Y. Kanda, K. Shimizu and K. Kato, ChemCatChem, 2018, 10, 2378–2382 CrossRef.
- J. Allan, Green Chem., 2013, 15, 596–600 RSC.
- Y. E. Jad, G. A. Acosta, S. N. Khattab, G. Beatriz, T. Govender, H. G. Kruger, A. El-Faham and F. Albericio, Org. Biomol. Chem., 2015, 13, 2393–2398 RSC.
- https://www.cdc.gov/niosh/docs/90-105/default.html.
- A. J. Parker, Q. Rev., Chem. Soc., 1962, 16, 163–187 RSC.
- S. Ding and N. Jiao, Angew. Chem., Int. Ed., 2012, 51, 9226–9237 CrossRef PubMed.
- V. Zadsirjan, M. Shiri, M. M. Heravi, T. Hosseinnejad, S. A. Shintre and N. A. Koorbanally, Res. Chem. Intermed., 2017, 43, 2119–2142 CrossRef.
- B. Baghernejad, M. Heravi, H. Oskooie, N. Poormohammad, M. Khorshidi and Y. Beheshtiha, Mol. Divers., 2011, 15, 245–248 CrossRef PubMed.
- T. Hosseinnejad, M. M. Heravi and R. Firouzi, J. Mol. Model., 2013, 19, 951–961 CrossRef PubMed.
- M. Jayanth, H. Naik, D. Tatke and S. Seshadri, Indian J. Chem., 1973, 11, 1112–1114 Search PubMed.
- S. Koeller and J.-P. Lellouche, Tetrahedron Lett., 1999, 40, 7043–7046 CrossRef.
- J.-P. Lellouche and S. Koeller, J. Org. Chem., 2001, 66, 693–696 CrossRef PubMed.
- M. Journet, D. Cai, L. M. DiMichele and R. D. Larsen, Tetrahedron Lett., 1998, 39, 6427–6428 CrossRef.
- D. S. Yang and H. B. Jeon, Bull. Korean Chem. Soc., 2010, 31, 1424–1426 CrossRef.
- D.-W. Gu and X.-X. Guo, Tetrahedron, 2015, 71, 9117–9122 CrossRef.
- Y. Oda, T. Sato and N. Chida, Org. Lett., 2012, 14, 950–953 CrossRef PubMed.
- S. Shirakawa, T. Tokuda, A. Kasai and K. Maruoka, Org. Lett., 2013, 15, 3350–3353 CrossRef PubMed.
- A. A. Wilson, J. W. Hicks, O. Sadovski, J. Parkes, J. Tong, S. Houle, C. J. Fowler and N. Vasdev, J. Med. Chem., 2012, 56, 201–209 CrossRef PubMed.
- L. Li, K.-C. Chang, Y. Zhou, B. Shieh, J. Ponder, A. D. Abraham, H. Ali, A. Snow, J. M. Petrash and D. V. LaBarbera, J. Med. Chem., 2013, 57, 71–77 CrossRef PubMed.
- C. Chen, L. Tan and P. Zhou, J. Saudi Chem. Soc., 2015, 19, 327–333 CrossRef.
- Z. Rappoport, Chemistry of the Cyano Group, John Wiley & Sons, London, 1970 Search PubMed.
- R. Larock, Comprehensive Organic Transformations: A Guide to Functional Group Preparations, John Wiley & Sons, New York, 1989 Search PubMed.
- J. S. Miller and J. L. Manson, Acc. Chem. Res., 2001, 34, 563–570 CrossRef PubMed.
- S. Nagamura, E. Kobayashi, K. Gomi and H. Saito, Bioorg. Med. Chem., 1996, 4, 1379–1391 CrossRef PubMed.
- A. Kleemann, J. Engel, B. Kutscher and D. Reichert, Pharmaceutical Substances; Syntheses, Patents, Applications, Thieme, Stuttgart, 2001 Search PubMed.
- M. Sundermeier, A. Zapf, M. Beller and J. Sans, Tetrahedron Lett., 2001, 42, 6707–6710 CrossRef.
- G. Ellis and T. Romney-Alexander, Chem. Rev., 1987, 87, 779–794 CrossRef.
- V. Grushin and H. Alper, Chem. Rev., 1994, 94, 1047–1062 CrossRef.
- P. Anbarasan, T. Schareina and M. Beller, Chem. Soc. Rev., 2011, 40, 5049–5067 RSC.
- Q. Wen, J. Jin, L. Zhang, Y. Luo, P. Lu and Y. Wang, Tetrahedron Lett., 2014, 55, 1271–1280 CrossRef.
- J. Kim, H. Kim and S. Chang, Org. Lett., 2012, 14, 3924–3927 CrossRef PubMed.
- B. Liu, J. Wang, B. Zhang, Y. Sun, L. Wang, J. Chen and J. Cheng, Chem. Commun., 2014, 50, 2315–2317 RSC.
- L. Zhang, Q. Wen, J. Jin, C. Wang, P. Lu and Y. Wang, Tetrahedron, 2013, 69, 4236–4240 CrossRef.
- S. Ding and N. Jiao, J. Am. Chem. Soc., 2011, 133, 12374–12377 CrossRef PubMed.
- J. Xiao, Q. Li, T. Chen and L.-B. Han, Tetrahedron Lett., 2015, 56, 5937–5940 CrossRef.
- S. Ushijima, K. Moriyama and H. Togo, Tetrahedron, 2011, 67, 958–964 CrossRef.
- A. B. Pawar and S. Chang, Chem. Commun., 2014, 50, 448–450 RSC.
- B. Venu, B. Vishali, G. Naresh, V. V. Kumar, M. Sudhakar, R. Kishore, J. Beltramini, M. Konarova and A. Venugopal, Catal. Sci. Technol., 2016, 6, 8055–8062 RSC.
- J. Kim, J. Choi, K. Shin and S. Chang, J. Am. Chem. Soc., 2012, 134, 2528–2531 CrossRef PubMed.
- Z. Wang and S. Chang, Org. Lett., 2013, 15, 1990–1993 CrossRef PubMed.
- K. Srinivas, E. B. Reddy and B. Das, Synlett, 2002, 2002, 0625–0627 CrossRef.
- W. Su, Y. Zhang, J. Li and P. Li, Org. Prep. Proced. Int., 2008, 40, 543–550 CrossRef.
- R. Gawley, The Beckmann Reactions: Rearrangements, Elimination–Additions, Fragmentations, and Rearrangement–Cyclizations, John Wiley & Sons, 1988 Search PubMed.
- Y. Izumi, S. Sato and K. Urabe, Chem. Lett., 1983, 12, 1649–1652 CrossRef.
- K. Narasaka, H. Kusama, Y. Yamashita and H. Sato, Chem. Lett., 1993, 22, 489–492 CrossRef.
- J. Yadav, B. Reddy, A. Madhavi and Y. Ganesh, J. Chem. Res., 2002, 2002, 236–238 CrossRef.
- Y. Ikushima, O. Sato, M. Sato, K. Hatakeda and M. Arai, Chem. Eng. Sci., 2003, 58, 935–941 CrossRef.
- M. Arisawa and M. Yamaguchi, Org. Lett., 2001, 3, 311–312 CrossRef PubMed.
- W. Su, Y. Zhang, J. Li and P. Li, Org. Prep. Proced. Int., 2008, 40, 543–550 CrossRef.
- H. Ucar and J. H. Poupaert, Heterocycles, 1997, 4, 805–810 CrossRef.
- M.-N. Zhao, R.-R. Hui, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2014, 16, 3082–3085 CrossRef PubMed.
- N. Candeias, L. Branco, P. Gois, C. Afonso and A. Trindade, Chem. Rev., 2009, 109, 2703–2802 CrossRef PubMed.
- X.-F. Wu, H. Neumann and M. Beller, Chem. Rev., 2012, 113, 1–35 CrossRef PubMed.
- J. Yuan, C. Liu and A. Lei, Chem. Commun., 2015, 51, 1394–1409 RSC.
- D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893–930 CrossRef PubMed.
- J. Asensio, E. Sánchez and P. Gómez-Romero, Chem. Soc. Rev., 2010, 39, 3210–3239 RSC.
- L. Carvalho, E. Fernandes and M. Marques, Chem.–Eur. J., 2011, 17, 12544–12555 CrossRef PubMed.
- S. Noël, S. Cadet, E. Gras and C. Hureau, Chem. Soc. Rev., 2013, 42, 7747–7762 RSC.
- N. P. Prajapati, R. H. Vekariya, M. A. Borad and H. D. Patel, RSC Adv., 2014, 4, 60176–60208 RSC.
- X. Shi, J. Guo, J. Liu, M. Ye and Q. Xu, Chem.–Eur. J., 2015, 21, 9988–9993 CrossRef PubMed.
- X. Gao, B. Yu, Q. Mei, Z. Yang, Y. Zhao, H. Zhang, L. Hao and Z. Liu, New J. Chem., 2016, 40, 8282–8287 RSC.
- J. Liu, H. Yi, X. Zhang, C. Liu, R. Liu, G. Zhang and A. Lei, Chem. Commun., 2014, 50, 7636–7638 RSC.
- S. Mondal, S. Samanta, S. Santra, A. K. Bagdi and A. Hajra, Adv. Synth. Catal., 2016, 358, 3633–3641 CrossRef.
- Y. Li, D. Xue, W. Lu, C. Wang, Z.-T. Liu and J. Xiao, Org. Lett., 2013, 16, 66–69 CrossRef PubMed.
- A. V. de Souza, G. F. Mendonça, R. B. Bernini and M. Mattos, J. Braz. Chem. Soc., 2007, 18, 1575–1579 CrossRef.
- W. Zeng, E. Wang, R. Qiu, M. Sohail, S. Wu and F.-X. Chen, J. Organomet. Chem., 2013, 743, 44–48 CrossRef.
- S.-F. Zhu, B. Xu, G.-P. Wang and Q.-L. Zhou, J. Am. Chem. Soc., 2011, 134, 436–442 CrossRef PubMed.
- S.-F. Zhu and Q.-L. Zhou, Acc. Chem. Res., 2012, 45, 1365–1377 CrossRef PubMed.
- L.-R. Wen, N.-N. Man, W.-K. Yuan and M. Li, J. Org. Chem., 2016, 81, 5942–5948 CrossRef PubMed.
- H. Yoshida, Y. Ito and J. Ohshita, Chem. Commun., 2011, 47, 8512–8514 RSC.
- S. V. Kessar, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Flemming, Pergamon Press, Oxford, 1991, vol. 4, pp. 483–515 Search PubMed.
- W. Liu, C. Chen and P. Zhou, J. Org. Chem., 2017, 82, 2219–2222 CrossRef PubMed.
- Q. Zhang, C. Song, H. Huang, K. Zhang and J. Chang, Org. Chem. Front., 2018, 5, 80–87 RSC.
- S. S. Priya, A. V. Nakhate, S. S. R. Gupta, K. B. Rasal, G. P. Deshmukh, C. R. Gadipelly, T. Srinivas, D. K. Dumbre, S. Periasamy and K. V. Chary, Appl. Catal., B, 2017 DOI:10.1016/j.apcatb.2017.12.058.
- K. Hosoi, K. Nozaki and T. Hiyama, Org. Lett., 2002, 4, 2849–2851 CrossRef PubMed.
- P. Fitton, M. Johnson and J. McKeon, Chem. Commun., 1968, 6–7 RSC.
- A. Kodimuthali, A. Mungara, P. L. Prasunamba and M. Pal, J. Braz. Chem. Soc., 2010, 21, 1439–1445 CrossRef.
- N. Iranpoor, H. Firouzabadi, Z. T. Rizi and S. Erfan, RSC Adv., 2014, 4, 43178–43182 RSC.
- W. Liu, C. Chen and H. Liu, Beilstein J. Org. Chem., 2015, 11, 1721 CrossRef PubMed.
- M. Sugiura, F. Robvieux and S. Kobayashi, Synlett, 2003, 2003, 1749–1751 Search PubMed.
- R. Adams and P. Dhull, J. Organomet. Chem., 2017, 849, 228–232 CrossRef.
- P. O. Nubel and T. L. Brown, J. Am. Chem. Soc., 1984, 106, 644–652 CrossRef.
- J. M. Robertson, I. W. Jones, K. M. Kayne, C. G. Contreras, D. J. Witter, R. B. Bates and H. Hall Jr, Tetrahedron Lett., 2011, 52, 6080–6081 CrossRef.
- D. J. Jung, H. J. Jeon, J. H. Kim, Y. Kim and S.-g. Lee, Org. Lett., 2014, 16, 2208–2211 CrossRef PubMed.
- P. Venkanna, K. Rajanna, M. S. Kumar, M. B. Ansari and M. M. Ali, Tetrahedron Lett., 2015, 56, 5164–5167 CrossRef.
- P. Venkanna, M. S. Kumar, K. C. Rajanna and M. M. Ali, Synth. React. Inorg. Met.-Org. Chem., 2015, 45, 97–103 CrossRef.
- C. M. Marson, Tetrahedron, 1992, 48, 3659–3726 CrossRef.
- C. Marson and P. Giles, Synthesis Using Vilsmeier Reagents. New Directions in Organic and Biological Chemistry, CRC Press, Boca Raton, FL, USA, 1994 Search PubMed.
- J. C. Jutz, Top. Curr. Chem., 1978, 73, 125–230 CrossRef.
- O. Meth-Cohn and B. Tarnowski, Adv. Heterocycl. Chem., 1982, 31, 207–236 CrossRef.
- A. Holý and Z. Arnold, Collect. Czech. Chem. Commun., 1965, 30, 53–60 CrossRef.
- A. R. Katritzky and C. M. Marson, J. Org. Chem., 1987, 52, 2726–2730 CrossRef.
- M. Sreenivasulu and K. G. Rao, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1989, 28, 494–495 Search PubMed.
- S. Selvi and P. T. Perumal, Synth. Commun., 2000, 30, 3925–3928 CrossRef.
- K. Jameleddine, H. A. M. Adnen and B. H. Béchir, Tetrahedron Lett., 2006, 47, 2973–2975 CrossRef.
- S. Megati and K. G. Rao, Tetrahedron Lett., 1995, 36, 5819–5822 CrossRef.
- B. Raju and G. S. Krishna Rao, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1987, 26B, 177 Search PubMed.
- B. Raju and G. S. Krishna Rao, Synthesis, 1985, 1985, 779–781 CrossRef.
- B. Raju and G. S. Krishna Rao, Synthesis, 1987, 1987, 197–199 CrossRef.
- M. Sreenivasulu and G. S. Krishna Rao, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1987, 26B, 1187 Search PubMed.
- B. Raju and G. S. Krishna Rao, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1987, 26B, 175 Search PubMed.
- A. D. Thomas and C. Asokan, Tetrahedron, 2004, 60, 5069–5076 CrossRef.
- W. Ziegenbein and W. Franke, Angew. Chem., 1959, 71, 573–574 CrossRef.
- Y. Y. Weng, J. J. Li and W. K. Su, Chin. Chem. Lett., 2011, 22, 1395–1398 CrossRef.
- W. Su, Z. Lu and X. Zhang, Org. Prep. Proced. Int., 2008, 40, 481–487 CrossRef.
- M. Kumar, K. Reddy, K. Rajanna, P. Venkanna and G. Krishnaiah, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2013, 43, 977–983 CrossRef.
- S. R. Narahari, B. R. Reguri and K. Mukkanti, Tetrahedron Lett., 2011, 52, 4888–4891 CrossRef.
- W.-C. Li, A.-H. Lu, R. Palkovits, W. Schmidt, B. Spliethoff and F. Schüth, J. Am. Chem. Soc., 2005, 127, 12595–12600 CrossRef PubMed.
- H. Ichihashi and M. Kitamura, Catal. Today, 2002, 73, 23–28 CrossRef.
- A. Fernández, M. Boronat, T. Blasco and A. Corma, Angew. Chem., Int. Ed., 2005, 44, 2370–2373 CrossRef PubMed.
- H. Moghanian, M. Shabanian and H. Jafari, C. R. Chim., 2012, 15, 346–349 CrossRef.
- E. Fischer, Ber. Dtsch. Chem. Ges., 1896, 29, 205–214 CrossRef.
- J. Cornforth and R. Cornforth, J. Chem. Soc., 1949, 0, 1028–1030 RSC.
- K. B. Rasal and G. D. Yadav, Catal. Today, 2017, 309, 51–60 CrossRef.
- J. Zhu, Z. Zhang, C. Miao, W. Liu and W. Sun, Tetrahedron, 2017, 73, 3458–3462 CrossRef.
- Y. Izumi and T. Fujita, J. Mol. Catal. A: Chem., 1996, 106, 43–49 CrossRef.
- T. Przewloka, S. Chen, Z. Xia, H. Li, S. Zhang, D. Chimmanamada, E. Kostik, D. James, K. Koya and L. Sun, Tetrahedron Lett., 2007, 48, 5739–5742 CrossRef.
- N. S. Mani, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1989, 28, 602–603 Search PubMed.
- A. Jarrahpour and M. Zarei, Tetrahedron Lett., 2009, 50, 1568–1570 CrossRef.
- B. Bhanage, S. Fujita, Y. Ikushima and M. Arai, Appl. Catal., A, 2001, 219, 259–266 CrossRef.
- H. Yasuda, L.-N. He, T. Takahashi and T. Sakakura, Appl. Catal., A, 2006, 298, 177–180 CrossRef.
- H. Yasuda, L.-N. He and T. Sakakura, J. Catal., 2002, 209, 547–550 CrossRef.
- N. Kihara, N. Hara and T. Endo, J. Org. Chem., 1993, 58, 6198–6202 CrossRef.
- M. Ratzenhofer and H. Kisch, Angew. Chem., Int. Ed., 1980, 19, 317–318 CrossRef.
- J. L. Jiang, Z. Xiu and R. Hua, Synth. Commun., 2008, 38, 232–238 CrossRef.
- P. Supsana, T. Liaskopoulosa, P. G. Tsoungas and G. Varvounis, Synlett, 2007, 2007, 2671–2674 CrossRef.
- M. Hoshi and K. Shirakawa, Tetrahedron Lett., 2000, 41, 2595–2598 CrossRef.
- P. Wu and J. Sun, Synth. Commun., 2008, 38, 1003–1010 CrossRef.
- I. Yavari, N. Hazeri, M. T. Maghsoodlou and A. Moradi, J. Chem. Res., 2001, 2001, 272–273 CrossRef.
- M. T. Maghsoodlou, N. Hazeri, S. M. Habibi-Khorassani, V. Solimani, G. Marandi and Z. Razmjoo, J. Chem. Res., 2008, 2008, 198–200 CrossRef.
- A. Aly, A. Mourad, A. Hassan, N. Mohamed, B. Ali and M. El-Sayed, Arch. Pharm., 2004, 337, 133–139 CrossRef PubMed.
- J. Clark-Lewis and M. Thompson, J. Chem. Soc., 1959, 0, 2401–2408 RSC.
- D. Pestov, V. Slesarev, A. Ginak and V. Slesareva, Chem. Heterocycl. Compd., 1988, 24, 782–786 CrossRef.
- H. Bredereck, R. Gompper, F. Effenberger, K. Hugo and G. Simchen, Chem. Ber., 1961, 94, 1241–1248 CrossRef.
- M. Sekiya and C. Yanaihara, Chem. Pharm. Bull., 1969, 17, 810–814 CrossRef.
- A. Hosomi, S. Kohra and Y. Tominaga, Chem. Commun., 1987, 0, 1517–1518 RSC.
- T. Hayashi, Y. Matsumoto, T. Kiyoi, Y. Ito, S. Kohra, Y. Tominaga and A. Hosomi, Tetrahedron Lett., 1988, 29, 5667–5670 CrossRef.
- A. Hosomi, S. Kohra, K. Ogata, T. Yanagi and Y. Tominaga, J. Org. Chem., 1990, 55, 2415–2420 CrossRef.
- A. S. Nagle, R. N. Salvatore, B.-D. Chong and K. W. Jung, Tetrahedron Lett., 2000, 41, 3011–3014 CrossRef.
- H. Aichaoui, J. Poupaert, D. Lesieur and J. Hénichart, Tetrahedron, 1991, 47, 6649–6654 CrossRef.
- H. Aichaoui, D. Lesieur and J. Hénichart, J. Heterocycl. Chem., 1992, 29, 171–175 CrossRef.
- S. Yous, J. H. Poupaert, I. Lesieur, P. Depreux and D. Lesieur, J. Org. Chem., 1994, 59, 1574–1576 CrossRef.
- A. A. Hullio, H. I. Afridi and G. Mastoi, Green Chem. Lett. Rev., 2017, 10, 274–284 CrossRef.
- J. Stille, Pure Appl. Chem., 1985, 57, 1771–1780 Search PubMed.
- M. Pereyre, J.-P. Quintard and A. Rahm, Tin in Organic Synthesis, Butterworths, London, 1987 Search PubMed.
- V. Farina, V. Krishnamurthy and W. Scott, The Stille Reaction, John Wiley, New York, 1997 Search PubMed.
- T. N. Mitchell, in Metal-Catalyzed Cross-Coupling Reactions, Second Edition, Wiley-VCH, Weinheim, 2004, pp. 125–161 Search PubMed.
- P. Espinet and A. Echavarren, Angew. Chem., Int. Ed., 2004, 43, 4704–4734 Search PubMed.
- G. Bringmann and D. Menche, Acc. Chem. Res., 2001, 34, 615–624 CrossRef PubMed.
- G. Bringmann, Y. Reichert and V. V. Kane, Tetrahedron, 2004, 60, 3539–3574 CrossRef.
- T. C. Roberts, P. A. Smith, R. T. Cirz and F. E. Romesberg, J. Am. Chem. Soc., 2007, 129, 15830–15838 CrossRef PubMed.
- T. W. Wallace, Org. Biomol. Chem., 2006, 4, 3197–3210 RSC.
- M. M. Heravi, E. Hashemi and F. Azimian, Tetrahedron, 2014, 1, 7–21 CrossRef.
- M. M. Heravi and L. Mohammadkhani, J. Organomet. Chem., 2018, 869, 106–200 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |