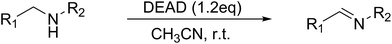Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of a metal-free amine oxidation method utilizing DEAD chemistry†
G. Wanga,
G. Piva de Silvab,
N. E. Wiebea,
G. M. Fehra and
R. L. Davis *a
*a
aDepartment of Chemistry, University of Manitoba, Winnipeg, Manitoba, Canada
bDepartamento de Química, Universidade Federal de São Carlos, São Carlos, Brazil. E-mail: Rebecca.Davis@umanitoba.ca
First published on 17th October 2017
Abstract
Herein, we examine the oxidative abilities of azodicarboxylates for the conversion of amines to imines. This method provides access to synthetically useful imine intermediates including β-carbolines, quinazolines and N-heterocyclic carbene precursors. The ability to recover spent azodicarboxylate for regeneration and further use underscores the applicability and appeal of this protocol.
Introduction
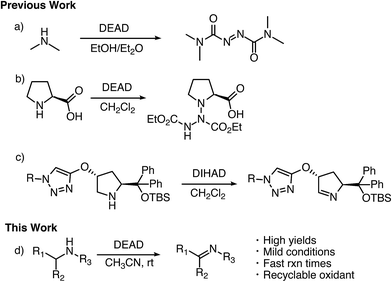
Imines serve as versatile building blocks in many synthetic transformations and, as a result, have been used in the construction of biologically active compounds, heterocycles, natural products, and agrochemicals.1 Traditional approaches for the formation of imines involve condensation of primary amines with carbonyls.2 This method suffers from limited scope and often requires unstable aldehydes and ketones. To overcome these issues, there has been a great deal of work devoted to developing amine dehydrogenation methods ranging from IBX oxidation to the bioinspired aerobic oxidation with transition metals.3 Despite these works, there is still a large demand for general oxidative methods for the conversion of amines to imines.Azodicarboxylates have long been employed as electrophilic species in synthetic transformations ranging from the Mitsunobu reaction4 and aminations5 to [4 + 2] cycloaddition reactions.6 They have also found application as ligands in the Cu catalyzed oxidation of alcohols and tetrahydraquinolines.7 However, the abilities of these electrophilic azo species to act as general and broadly applicable oxidizing agents for amines has yet to be well documented or examined.8
In the mid 1900's, the examination of the reactivity of azodicarboxylates with aliphatic primary and secondary amines suggested they primarily provided substituted amides (Scheme 1a).9 The most recent data on the reactivity of azodicarboxylates with amines comes from organocatalytic amination reactions where the azodicarboxylates often undergo side reactions with secondary amine catalysts. For example, it has been shown that L-proline reacts with diethyl azodicarboxylate (DEAD) to form a triazane species (Scheme 1b) and that diisopropyl azodicarboxylate (DIAD) is capable of deactivating a Jørgensen–Hayashi catalyst containing a tethered base (Scheme 1c).10,11 The ability of azodicarboxylates to oxidize amines as reported by Pericàs and as observed by our group in the development of other organocatalytic amination reactions has led us to explore the oxidizing abilities of azodicarboxylates. Herein we describe an efficient, atom economical, and general method for the oxidation of amines to imines under mild conditions using DEAD (Scheme 1d).
Results and discussion
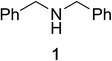
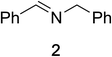
Initial studies on the oxidative abilities of azo compounds revealed that secondary amines could be readily oxidized by DEAD to form the corresponding imines. Reaction of dibenzylamine 1 with DEAD in CDCl3 provided nearly quantitative conversion to dibenzylimine 2 in 1.5 h (Table 1, entry 1). The reduction of DEAD to diethyl hydrazodicarboxylate was also observed in this reaction. In an attempt to improve upon these initial findings, a screening of reaction conditions was performed.12 The reaction was found to give high conversions in all solvents. In general, polar solvents provided faster conversion than non-polar solvents with acetonitrile providing the cleanest conversion (Table 1, entry 5).13,14The oxidative abilities of various azo compounds were also explored (Table 1, entries 5–9). DEAD provided the highest conversion (99%), while DIAD and 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) gave relatively high conversion (91% and 94% respectively). No conversion was observed when AIBN or 4-nitroazobenzene (NAB) was employed as the oxidant. This suggests that an electrophilic azo species is required for the oxidation. Consequently, acetonitrile and DEAD were selected to explore the scope of this oxidative methodology. Using these conditions, we established the ability of this reaction to proceed on a larger scale (4.00 mmol 1) with full conversion (see ESI for details†).
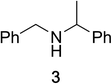
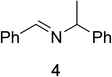
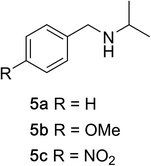
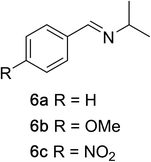
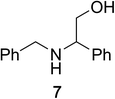
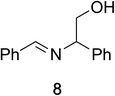
To determine the effectiveness of this oxidative method over a range of amines we examined alkylbenzylamines and dibenzylamines (Table 2). In most cases we observed high yields and full selectivity within 90 min. Full regioselectivity was observed for the secondary carbon of 3 and 5a–c. Electron donating and electron withdrawing groups on the aromatic moiety had no significant influence on the yield or selectivity of the reaction (5b and 5c). The amino alcohol 7 was converted to 8 with full chemoselectivity for the amine. Reactions between DEAD and amines 3, 5, and 7 demonstrate that less hindered sites are oxidized preferentially, suggesting a kinetic driving force.
| Substrate | Producta | Yieldb (%) | Time (min) |
|---|---|---|---|
| a 0.20 mmol amine in 0.5 mL of CH3CN, 1.2 eq. DEAD.b Reported yields after purification by flash chromatography on silica.c Yields measured by 1H NMR, hexamethyldisilane as internal standard.d In refluxing acetonitrile.e 2.2 eq. DEAD.f 21 observed in 14% yield at 20 min. | |||
 |
 |
96c | 90 |
 |
 |
95c | 90 |
 |
 |
95c | 90 |
| 88c | 90 | ||
| 90c | 90 | ||
 |
 |
82c | 90 |
 |
 |
85c,d | 90 |
 |
 |
98 | 20 |
 |
 |
77 | 20 |
 |
 |
84c,e,f | 20 |
 |
 |
95e | 50 |
| 90e | 50 | ||
| 95e | 50 | ||
| 80e | 50 | ||
 |
 |
78 | 60 |
| 82 | 60 | ||
| 70 | 60 | ||
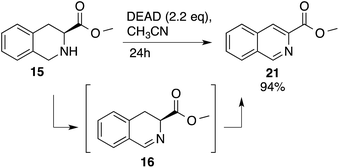
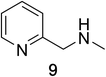
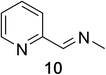
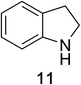
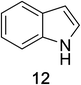
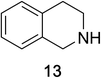
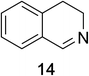
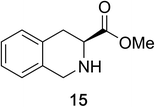
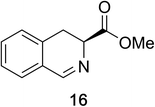
Heteroaromatic amine 9 was found to provide the desired imine 10 in 85% yield in 90 min. To achieve the imine as the major product this reaction was conducted in refluxing acetonitrile, as lower temperatures resulted in a mixture of triazane species. Heterocyclic compounds 11 and 13 achieved >99% conversion within 20 min. The corresponding products were isolated in good to excellent yields (Table 2).15 Methyl tetrahydroisoquinolinecarboxylate (15) was employed to examine the selectivity between alpha- and benzyl- positions. At 20 min starting material had been consumed and imine 16 was observed in 84% yield with 14% of aromatized product 21 also present. Compound 21 was isolated in 94% yield after 24 hours (Scheme 2).
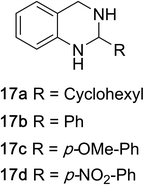
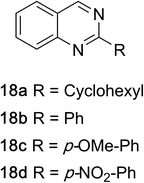
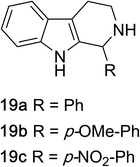
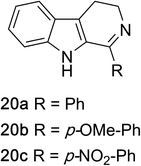
1,2,3,4-Tetrahydroquinazolines with both aryl and alkyl substituents at the 2-position (Table 2, 17a–d) were found to readily undergo double oxidation to aromatic quinazoline derivatives 18a–d with 2.2 equivalents of DEAD. In all cases high conversions and isolated yields were obtained. Phenyl substituted tetrahydro-β-carboline 19a provided the corresponding imine in >99% conversion and was isolated in 78% yield. β-Carboline derivatives containing electron donating and withdrawing groups showed equivalent conversions in 1 hour; however, isolated yields inversely correlate with the imine lability.
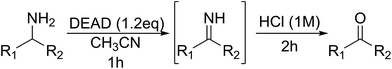
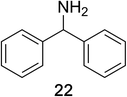
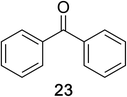
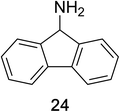
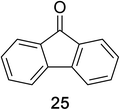
Encouraged by the broad scope of secondary amines that could be applied, we sought to extend the methodology to primary amines. Bulky benzyl amines 22 and 24 were found to favour amine oxidation over carboxylate substitution. Due to the difficulty associated with isolation of these labile primary imines, the species were hydrolyzed in situ and isolated as the corresponding ketones. The resulting products were successfully isolated in moderate yields (Table 3).
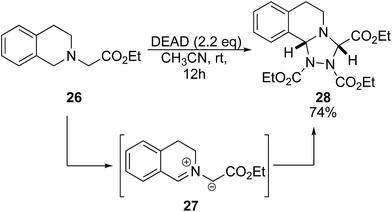
Previous studies of DEAD and tertiary amines show general dealkylation.6 However, given the success of benzyl amines and α-amino esters, we envisioned that the presence of an acidic proton on the tertiary amine would promote oxidation over dealkylation. When ethyl 3,4-dihydro-2(1H)-isoquinolinylacetate 26 was reacted with 2.2 eq. of DEAD, we observed production of triazolidine 28 (Scheme 3).16 This product was likely obtained via a 1,3-dipolar cycloaddition of the azomethine ylide intermediate 27 with DEAD.17 Scaffolds such as this are well known as antifungal drugs for medical and preservative purposes.18 It is envisioned that this method could also serve to generate precursors for asymmetric N-heterocyclic carbenes.19
A key feature of this oxidation process is the ease of recovery of the spent oxidizing agent, hydrazodicarboxlate. This compound is insoluble in non-polar solvents and can be readily separated from the imine via crystallization from toluene. This process allows for 80% recovery of diethylhydrazodicarboxlate, which can be readily reoxidized to DEAD.
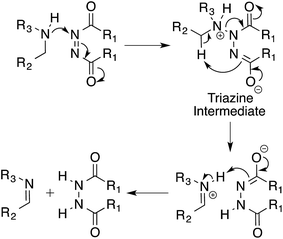
Based on our experimental results we propose a mechanism for the oxidation of amines by azodicarboxylates via a triazane intermediate (Scheme 4).20 In this triazane pathway mechanism, the first step involves the nucleophilic attack of the amine on DEAD, taking advantage of the electrophilic nature of the azo species. The resulting triazane intermediate then undergoes an intramolecular elimination and subsequent proton transfer to produce the imine product. The details of this mechanism are currently under investigation.
Conclusions
In this work we have described the first definitive demonstration of the oxidative abilities of azodicarboxylates with amines, greatly expanding their applicability in organic chemistry. This method not only provides access to versatile imine building blocks, but it also affords a rapid synthetic route to key alkaloid intermediates (e.g. β-carbolines, quinazolines) used across pharmaceutical and agrochemical synthesis. Additionally, the tandem oxidation/1,3-dipolar cycloaddition provides access to biologically relevant triazole scaffolds that may also serve as novel NHC scaffolds. The applicability of this reaction is accentuated by the ability to recover the spent oxidizing agent for regeneration and further use.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) P. D. Bailey and K. M. Morgan, Organonitrogen Chemistry, Oxford University Press, New York, 1996 Search PubMed; (b) S. Patai, The Chemistry of the Carbon Nitrogen Double Bond, Chemistry of Functional Groups, Wiley_Interscience, New York, 1970 Search PubMed; (c) S. F. Martin, Pure Appl. Chem., 2009, 81, 195 CrossRef CAS PubMed; (d) D. R. Baker, J. G. Fenyes and G. S. Basarab, Synthesis and Chemistry of Agrochemicals IV. ACS Symposium Series, American Chemical Society, Washington D.C, 1995, vol. 584 Search PubMed.
- (a) E. J. Corey and K. Achiwa, J. Am. Chem. Soc., 1969, 91, 1429 CrossRef CAS; (b) A. L. Korich and T. S. Hughes, Synlett, 2007, 2602 CAS; (c) S. Morales, F. G. Guijarro, J. L. G. Ruano and M. B. Cid, J. Am. Chem. Soc., 2014, 136, 1082 CrossRef CAS PubMed; (d) M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 2003 RSC.
- (a) A. E. Wendlandt and S. S. Stahl, J. Am. Chem. Soc., 2014, 136, 506 CrossRef CAS PubMed; (b) E. Zhang, H. Tian, S. Xu, X. Yu and Q. Xu, Org. Lett., 2013, 15, 2704 CrossRef CAS PubMed; (c) A. E. Wendlandt and S. S. Stahl, Org. Lett., 2012, 14, 2850 CrossRef CAS PubMed; (d) J. S. M. Samec, A. H. Éll and J.-E. Bäckvall, Chem.–Eur. J., 2005, 11, 2327 CrossRef CAS PubMed; (e) K. C. Nicolaou, C. J. N. Mathison and T. Montagnon, J. Am. Chem. Soc., 2004, 126, 5192 CrossRef CAS PubMed; (f) K. C. Nicolaou, T. Montagnon, P. S. Baran and Y.-L. Zhong, J. Am. Chem. Soc., 2002, 124, 2245 CrossRef CAS PubMed; (g) For a recent review on amine oxidation, see: B. Chen, L. Wang and S. Gao, ACS Catal., 2015, 5, 5851 CrossRef CAS.
- K. C. K. Swamy, N. N. B. Kumar, E. Balaraman and K. V. P. P. Kumar, Chem. Rev., 2009, 109, 2551 CrossRef CAS PubMed.
- For selected examples of amination reactions employing DEAD, see; (a) D. A. Evans and S. G. Nelson, J. Am. Chem. Soc., 1997, 119, 6452 CrossRef CAS; (b) D. A. Evans and D. S. Johnson, Org. Lett., 1999, 1, 595 CrossRef CAS PubMed; (c) B. List, J. Am. Chem. Soc., 2002, 124, 5656 CrossRef CAS PubMed; (d) S. Bertelsen, M. Marigo, S. Brandes, P. Dinér and K. A. Jørgensen, J. Am. Chem. Soc., 2006, 128, 12973 CrossRef CAS PubMed.
- (a) G. Desimoni, G. Faita, P. P. Righetti and L. Toma, Tetrahedron, 1990, 46, 7951 CrossRef CAS; (b) G. Jenner and R. B. Salem, J. Chem. Soc., Perkin Trans. 2, 1990, 1961 RSC; (c) G. Desimoni, G. Faita, P. P. Righetti, A. Sfulcini and D. Tsyganov, Tetrahedron, 1994, 50, 1821 CrossRef CAS; (d) M. R. Gholami and A. Habibi Yangjeh, J. Phys. Org. Chem., 2000, 13, 468 CrossRef CAS; A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 Search PubMed.
- (a) S. D. McCann and S. S. Stahl, J. Am. Chem. Soc., 2016, 138, 199 CrossRef CAS PubMed; (b) D. Jung, M. H. Kim and J. Kim, Org. Lett., 2016, 18, 6300 CrossRef CAS PubMed.
- For examples of DEAD used in oxidation of amines: (a) J. Kroutil, T. Trnka and M. Cerny, Org. Lett., 2000, 2, 1681 CrossRef CAS PubMed; (b) J. Kroutil, T. Trnka and M. Cerny, Synthesis, 2004, 446 CrossRef CAS; (c) X. Xu, X. Li, L. Ma, N. Ye and B. Weng, J. Am. Chem. Soc., 2008, 130, 14048 CrossRef CAS PubMed; (d) M. Stone, Org. Lett., 2011, 13, 2326 CrossRef CAS PubMed.
- (a) E. E. Smissman and A. Makriyannis, J. Org. Chem., 1973, 38, 1652 CrossRef CAS PubMed; (b) F. R. Farr and W. S. Wadsworth, Proc. S. D. Acad. Sci., 1968, 47, 192 CAS.
- (a) J. E. Hein, A. Armstrong and D. G. Blackmond, Org. Lett., 2011, 13, 4300 CrossRef CAS PubMed; (b) T. Kanzian and H. Mayr, Chem.–Eur. J., 2010, 16, 11670 CrossRef CAS PubMed.
- X. Fan, S. Sayalero and M. A. Pericàs, Adv. Synth. Catal., 2012, 354, 2971 CrossRef CAS.
- No change in conversion was observed when the reaction was carried out under argon (Table 1, entry 2), eliminating the possibility of oxygen acting as a co-oxidant.
- Alcohols were not used as solvents because it was known that alcohols can be oxidized in the presence of DEAD. See ref. 10.
- A triazane species was observed as a minor side product (<2%) in CDCl3 but was absent in CD3CN.
- Isolated yields were poor in comparison to NMR conversion due to degradation of the imine on silica during purification.
- The relative steoreochemistry was determined by NOE experiments. For more details, see ESI.†.
- For recent reviews in 1,3-dipolar cycloaddition with azomethine ylide see: (a) I. Coldham and R. Hufton, Chem. Rev., 2005, 105, 2765 CrossRef CAS PubMed; (b) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366 CrossRef CAS PubMed.
- C. Lass-Flörl, Drugs, 2011, 71, 2405 CrossRef PubMed.
- G.-T. Li, G. Qing and S.-L. You, Chem. Sci., 2015, 6, 4273 RSC.
- The existence of a triazane species is supported by previous reports on the reaction of L-proline with azodicarboxylates. See ref. 10.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, analytical data, and NMR spectra. See DOI: 10.1039/c7ra09165f |
| This journal is © The Royal Society of Chemistry 2017 |