Themed collection Editors' Collection: Greener synthetic approaches towards quinoline derivatives

Microwave-assisted multicomponent synthesis of antiproliferative 2,4-dimethoxy-tetrahydropyrimido[4,5-b]quinolin-6(7H)-ones
Herein, we demonstrate a simple, rapid and green synthesis of 2,4-dimethoxy-THPQs under microwave irradiation and their antiproliferative activity, in silico ADMET and drug-likeness studies were carried out.
![Graphical abstract: Microwave-assisted multicomponent synthesis of antiproliferative 2,4-dimethoxy-tetrahydropyrimido[4,5-b]quinolin-6(7H)-ones](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D2RA04669E&imageInfo.ImageIdentifier.Year=2022)
RSC Adv., 2022,12, 30404-30415
https://doi.org/10.1039/D2RA04669E
Environmentally friendly domino multicomponent strategy for the synthesis of pyrroloquinolinone hybrid heterocycles
An efficient and elegant assembly of pyrene/aryl fused pyrrolo[2,3-b]quinolinone and pyrrolizino[3,2-b]quinolinone hybrid heterocycles was achieved via a domino multicomponent reaction strategy using a solid state melt reaction (SSMR) condition.

RSC Adv., 2022,12, 15440-15446
https://doi.org/10.1039/D2RA02851D
Synthesis and characterization of novel hercynite@sulfuric acid and its catalytic applications in the synthesis of polyhydroquinolines and 2,3-dihydroquinazolin-4(1H)-ones
Herein, we report the synthesis of hercynite@sulfuric acid as a novel nanomagnetic solid acid catalyst, containing the sulfuric acid catalytic sites on the surface of hercynite MNPs as the catalytic support.

RSC Adv., 2022,12, 2770-2787
https://doi.org/10.1039/D1RA07381H
Synthesis of indeno-[1,2-b]-quinoline-9,11(6H,10H)-dione and 7,7-dimethyl-10-aryl-7,8-dihydro-5H-indeno[1,2-b]quinoline-9,11(6H,10H)-dione derivatives in presence of heterogeneous Cu/zeolite-Y as a catalyst
A simple synthesis of indeno-[1,2-b]-quinoline-9,11-(6H,10H)-dione derivatives and 7,7-dimethyl-10-aryl-7,8-dihydro-5H-indeno[1,2-b]quinoline-9,11(6H,10H)-diones using aromatic aldehydes with heterogeneous CuO on a zeolite-Y catalyst is reported.
![Graphical abstract: Synthesis of indeno-[1,2-b]-quinoline-9,11(6H,10H)-dione and 7,7-dimethyl-10-aryl-7,8-dihydro-5H-indeno[1,2-b]quinoline-9,11(6H,10H)-dione derivatives in presence of heterogeneous Cu/zeolite-Y as a catalyst](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1RA06637D&imageInfo.ImageIdentifier.Year=2022)
RSC Adv., 2022,12, 2083-2093
https://doi.org/10.1039/D1RA06637D
Ultrasound assisted synthesis of hybrid quinoline-imidazole derivatives: a green synthetic approach
A green, straightforward and efficient study for obtaining hybrid quinoline-imidazole derivatives under ultrasound (US) irradiation as well as under conventional thermal heating (TH) has been presented.

RSC Adv., 2021,11, 38297-38301
https://doi.org/10.1039/D1RA07484A
Engineered N-doped graphene quantum dots/CoFe2O4 spherical composites as a robust and retrievable catalyst: fabrication, characterization, and catalytic performance investigation in microwave-assisted synthesis of quinoline-3-carbonitrile derivatives
Sustainable fabrication of spherical N-GQDs/CoFe2O4 nanocomposites as a novel magnetically retrievable catalyst for the synthesis of quinoline-3-carbonitrile derivatives has been developed.

RSC Adv., 2021,11, 34724-34734
https://doi.org/10.1039/D1RA05739A
Cesium salt of 2-molybdo-10-tungstophosphoric acid as an efficient and reusable catalyst for the synthesis of uracil derivatives via a green route
A solid catalyst, cesium salt of 2-molybdo-10-tungstophosphoric acid (Cs2.3H0.7PW10Mo2O40) named as Cs-3, was synthesized by a simple, cheap, clean, and eco-friendly method.

RSC Adv., 2021,11, 33980-33989
https://doi.org/10.1039/D1RA05190C
Synthesis of 2-ethoxycarbonylthieno[2,3-b]quinolines in biomass-derived solvent γ-valerolactone and their biological evaluation against protein tyrosine phosphatase 1B
A series of 2-ethoxycarbonylthieno[2,3-b]quinolines were synthesized in the bio-derived “green” solvent γ-valerolactone and evaluated for their inhibitory activities against PTP1B, compound 6a displayed an IC50 value of 8.04 ± 0.71 μM with 4.34-fold preference over TCPTP.
![Graphical abstract: Synthesis of 2-ethoxycarbonylthieno[2,3-b]quinolines in biomass-derived solvent γ-valerolactone and their biological evaluation against protein tyrosine phosphatase 1B](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0RA09247A&imageInfo.ImageIdentifier.Year=2021)
RSC Adv., 2021,11, 3216-3220
https://doi.org/10.1039/D0RA09247A
In situ synthesis of SO3H supported Fe3O4@resorcinol–formaldehyde resin core/shell and its catalytic evaluation towards the synthesis of hexahydroquinoline derivatives in green conditions
A novel core@double-shell acidic nanocatalyst (Fe3O4@SiO2@RF–SO3H) was prepared, characterized and applied in catalytic one-pot condensation between aromatic aldehydes, dimedone, malononitrile, and ammonium acetate for synthesis of hexahydroquinoline derivatives.

RSC Adv., 2020,10, 41703-41712
https://doi.org/10.1039/D0RA06972H
Core–shell magnetic mesoporous N-doped silica nanoparticles: solid base catalysts for the preparation of some arylpyrimido[4,5-b]quinoline diones under green conditions
Preparation of core–shell magnetic mesoporous N-doped silica nanoparticles as a new solid base catalyst was studied. obtained catalyst was used for the preparation of some arylpyrimido[4,5-b]quinoline diones under green conditions.
![Graphical abstract: Core–shell magnetic mesoporous N-doped silica nanoparticles: solid base catalysts for the preparation of some arylpyrimido[4,5-b]quinoline diones under green conditions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0RA06546C&imageInfo.ImageIdentifier.Year=2020)
RSC Adv., 2020,10, 35397-35406
https://doi.org/10.1039/D0RA06546C
A novel substrate directed multicomponent reaction for the syntheses of tetrahydro-spiro[pyrazolo[4,3-f]quinoline]-8,5′-pyrimidines and tetrahydro-pyrazolo[4,3-f]pyrimido[4,5-b]quinolines via selective multiple C–C bond formation under metal-free conditions
Substrate selectivity in the novel multi-component reaction of 5-aminoindazole, barbituric acid derivatives and aldehyde is explored.
![Graphical abstract: A novel substrate directed multicomponent reaction for the syntheses of tetrahydro-spiro[pyrazolo[4,3-f]quinoline]-8,5′-pyrimidines and tetrahydro-pyrazolo[4,3-f]pyrimido[4,5-b]quinolines via selective multiple C–C bond formation under metal-free conditions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0RA02990D&imageInfo.ImageIdentifier.Year=2020)
RSC Adv., 2020,10, 19600-19609
https://doi.org/10.1039/D0RA02990D
Malononitrile-activated synthesis and anti-cholinesterase activity of styrylquinoxalin-2(1H)-ones
SQs displaying anti-Alzheimer activity is serendipitous. Malononitrile as a handle to facilitate nucleophilic attack has been applied for the first time for the easy access of SQs.
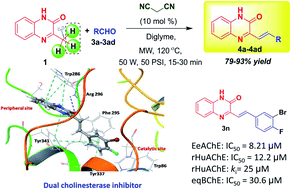
RSC Adv., 2020,10, 15966-15975
https://doi.org/10.1039/D0RA02816A
Iodine-catalyzed convergent aerobic dehydro-aromatization toward benzazoles and benzazines
An iodine-catalyzed aerobic dehydro-aromatization has been developed, providing a straightforward and efficient access to various benzoazoles and benzoazines.
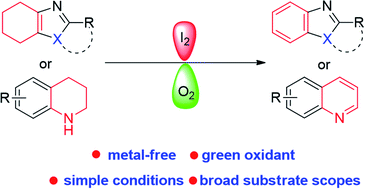
RSC Adv., 2020,10, 8348-8351
https://doi.org/10.1039/C9RA10964A
Regioselective ring expansion followed by H-shift of 3-ylidene oxindoles: a convenient synthesis of N-substituted/un-substituted pyrrolo[2,3-c] quinolines and marinoquinolines
Herein, we report a simple and metal-free protocol for the synthesis of 4-oxo-4,5-dihydro-3H-pyrrolo[2,3-c]quinolines.
![Graphical abstract: Regioselective ring expansion followed by H-shift of 3-ylidene oxindoles: a convenient synthesis of N-substituted/un-substituted pyrrolo[2,3-c] quinolines and marinoquinolines](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9RA07831B&imageInfo.ImageIdentifier.Year=2019)
RSC Adv., 2019,9, 35068-35072
https://doi.org/10.1039/C9RA07831B
A multicomponent, facile and catalyst-free microwave-assisted protocol for the synthesis of pyrazolo-[3,4-b]-quinolines under green conditions
A facile, swift and ecofriendly microwave-assisted multi-component/one-pot protocol is designed for the synthesis of novel pyrazolo-[3,4-b]-quinolines at ambient temperature in aqueous ethanol as a reaction medium.
![Graphical abstract: A multicomponent, facile and catalyst-free microwave-assisted protocol for the synthesis of pyrazolo-[3,4-b]-quinolines under green conditions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9RA04604F&imageInfo.ImageIdentifier.Year=2019)
RSC Adv., 2019,9, 30768-30772
https://doi.org/10.1039/C9RA04604F
KOtBu-promoted oxidative dimerizations of 2-methylquinolines to 2-alkenyl bisquinolines with molecular oxygen
KOtBu-promoted oxidative dimerizations of 2-methylquinolines with molecular oxygen as the oxidant have been developed for the first time.

RSC Adv., 2019,9, 30139-30143
https://doi.org/10.1039/C9RA06465F
An efficient nickel/silver co-catalyzed remote C–H amination of 8-aminoquinolines with azodicarboxylates at room temperature
C–H amination at the C5 position of 8-aminoquinolines with azodicarboxylates proceeded efficiently using a nickel/silver co-catalyst at room temperature without any additional ligand, base or oxidant.
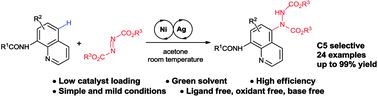
RSC Adv., 2018,8, 37064-37068
https://doi.org/10.1039/C8RA07647B
The synthesis of imidazo[1,5-a]quinolines via a decarboxylative cyclization under metal-free conditions
An iodine-mediated decarboxylative cyclization was developed from α-amino acids and 2-methyl quinolines under metal-free conditions, affording a variety of imidazo[1,5-a]quinolines with moderate to good yields.
![Graphical abstract: The synthesis of imidazo[1,5-a]quinolines via a decarboxylative cyclization under metal-free conditions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8RA03786H&imageInfo.ImageIdentifier.Year=2018)
RSC Adv., 2018,8, 23058-23065
https://doi.org/10.1039/C8RA03786H
Triple zirconocene/brønsted acid/CuO cooperative and relay catalysis system for tandem Mannich addition/C–C formative cyclization/oxidation
Triple zirconocene/brønsted acid/CuO cooperative and relay catalysis system for tandem Mannich addition/C–C formative cyclization/oxidation.
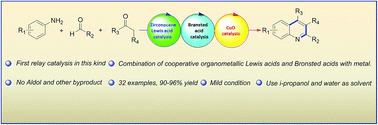
RSC Adv., 2017,7, 28616-28625
https://doi.org/10.1039/C7RA00870H
Highly efficient one-pot tandem Friedlander annulation and chemo-selective Csp3–H functionalization under calcium catalysis
Highly efficient and regioselective Friedlander synthesis of 2-methyl-3-acyl quinolines and their chemoselective Csp3–H functionalization is described for the first time using Ca(OTf)2 as the sustainable catalyst under solvent free conditions with atom and step economy.

RSC Adv., 2017,7, 18874-18882
https://doi.org/10.1039/C6RA28642A
High-efficiency catalytic performance over mesoporous Ni/beta zeolite for the synthesis of quinoline from glycerol and aniline
Quinoline was synthesized via the typical Skraup approach with a vapor-phase process. The mesoporous Ni/beta zeolite catalyst exhibited high-efficiency catalytic activity and an enhanced ability of anti-deactivation.
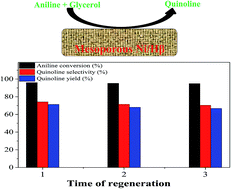
RSC Adv., 2017,7, 9551-9561
https://doi.org/10.1039/C6RA26736J
About this collection
RSC Advances is delighted to announce a new web collection titled ‘Greener synthetic approaches towards quinoline derivatives’. This collection is Edited by the Associate Editor Dr. Manojit Pal, Senior Professor, Dr Reddy’s Institute of Life Sciences, University of Hyderabad Campus, Hyderabad, India.
Background and scope
Quinoline, being a versatile and privileged framework in pharmaceutical sciences, drug discovery and development is part of building blocks for several marketed antimalarial drugs. Quinolines also display antimicrobial, analgesic, cardiovascular, anticancer and anti-inflammatory activities and are widespread in nature especially in alkaloids. It is therefore not surprising that over the years quinolines have attracted continued interest in the area of organic synthesis. While enormous efforts have been devoted for the development of elegant synthetic routes to various quinoline derivatives several of them are not eco-friendly in nature. On the other hand, the green or environmentally friendly synthetic approaches are in great demand in organic synthesis because they decrease or eliminate the usage or formation of harmful substances thereby preventing the environmental pollution considerably. Thus development of greener or eco-friendly approaches for useful compounds or agents including quinolines is an important goal. The current web collection is mainly a compilation of relevant important and interesting research papers (but no review articles) already published in RSC Advances during last 7 years. The major focus of this compilation was on selection of the greener or environmentally friendly synthetic methods including single or multi-step approaches, multi-component reactions, catalysed reactions including C-H activation, metal-free methods, microwave or ultrasound-assisted reactions etc reported for quinoline derivatives. Papers describing the eco-friendly synthesis along with biological evaluation of quinoline derivatives are also included.
RSC Advances now welcome research papers on new and latest developments in the area of eco-friendly synthesis of quinolines for further inclusion in the web collection.