Themed collection Celebrating the 80th Birthday of Professor Ei-ichi Negishi

In celebration of the 80th birthday of Professor Ei-ichi Negishi
Photo credit © Steve Scherer, Purdue University.

Org. Chem. Front., 2015,2, 872-873
https://doi.org/10.1039/C5QO90033F
Silylative coupling of olefins with vinylsilanes in the synthesis of functionalized alkenes
Recent developments in the sequential synthetic strategies including ruthenium-catalyzed silylative coupling followed by desilylation, leading to π-conjugated organic derivatives are presented.
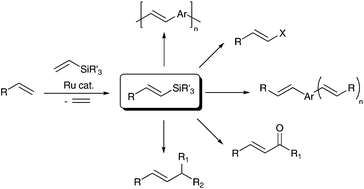
Org. Chem. Front., 2015,2, 730-738
https://doi.org/10.1039/C5QO00018A
Negishi coupling in the synthesis of advanced electronic, optical, electrochemical, and magnetic materials
Negishi coupling is an efficient and versatile tool for selective C–C bond formation in the synthesis of organic electronic, optical, electrochemical, and magnetic materials.
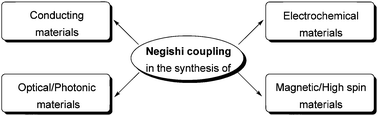
Org. Chem. Front., 2015,2, 416-445
https://doi.org/10.1039/C4QO00322E
Conquering three-carbon axial chirality of allenes
Axially chiral allenes are an important class of molecules widely existing in nature and also valuable chiral building blocks for organic synthesis. With more and more new protocols for racemic allenes and new chiral ligands being developed, a wide variety of novel and challenging transformations have been recently unveiled for the synthesis of this type of highly versatile compounds. In this review, we present a critical account of the enantioselective synthesis of axially chiral allenes.
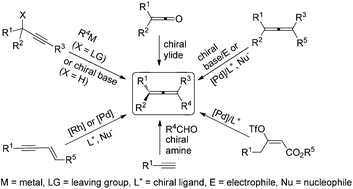
Org. Chem. Front., 2014,1, 1210-1224
https://doi.org/10.1039/C4QO00208C
Organometallic intermediate-based organic synthesis: organo-di-lithio reagents and beyond
Metal-mediated organic reactions have become one of the great frontiers of organic synthesis.
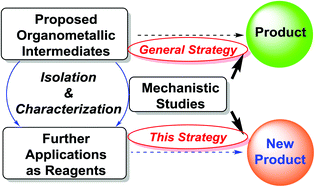
Org. Chem. Front., 2014,1, 1132-1139
https://doi.org/10.1039/C4QO00231H
A novel dynamic pseudo[1]rotaxane based on a mono-biotin-functionalized pillar[5]arene
A stable pillar[5]arene-based pseudo[1]rotaxane P1′ was synthesized by the click reaction, which exhibited a dynamic slow disassembly process upon adding a strong-polar solvent or competitive guest. Moreover, this dynamic behavior might be used as a switch to turn on or off the bioactivity of the biotin moiety in aqueous solution.
![Graphical abstract: A novel dynamic pseudo[1]rotaxane based on a mono-biotin-functionalized pillar[5]arene](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5QO00159E&imageInfo.ImageIdentifier.Year=2015)
Org. Chem. Front., 2015,2, 1013-1017
https://doi.org/10.1039/C5QO00159E
Regio- and stereoselective multisubstituted olefin synthesis via hydro/carboalumination of alkynes and subsequent iron-catalysed cross-coupling reaction with alkyl halides
A new synthetic route towards multisubstituted olefins was developed based on the direct cross coupling of alkenyl aluminium reagents, prepared by hydro- and carboalumination, with alkyl halides in the presence of an iron catalyst.

Org. Chem. Front., 2015,2, 1053-1058
https://doi.org/10.1039/C5QO00147A
Asymmetric synthesis of γ-aryl-substituted GABA derivatives via a highly diastereoselective Rh-catalyzed boronic acid addition at room temperature
A highly diastereoselective Rh-catalyzed boronic acid addition to enantiopure sulfinylimines at room temperature providing γ-aryl GABA derivatives has been described.
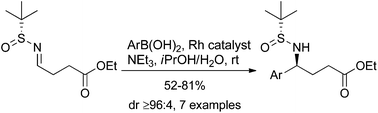
Org. Chem. Front., 2015,2, 885-889
https://doi.org/10.1039/C5QO00133A
Nickel-catalyzed decarboxylative arylation of azoles with perfluoro- and nitrobenzoates
This manuscript details a nickel-catalyzed method for the arylation of azole C–H bonds using perfluoro- and 2-nitro benzoates.
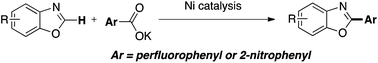
Org. Chem. Front., 2015,2, 726-729
https://doi.org/10.1039/C5QO00040H
Synthesis of morpholine or piperazine derivatives through gold-catalyzed cyclization reactions of alkynylamines or alkynylalcohols
A gold catalyzed reaction for the preparation of morpholine and piperazine derivatives from alkynylalcohols or alkynylamines was developed.
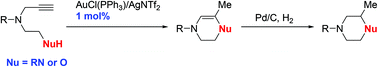
Org. Chem. Front., 2015,2, 721-725
https://doi.org/10.1039/C5QO00060B
Sulfur-containing stable five-membered “cycloallene” complexes: 1-thia-2-zircona- and 1-thia-2-titanacyclopenta-3,4-dienes
Stable five-membered sulfur-containing metallacyclic allenes, 1-thia-2-metallacyclopenta-3,4-diene, were synthesized from the reactions of low-valent zirconocene or titanocene with alkynylthioamides.
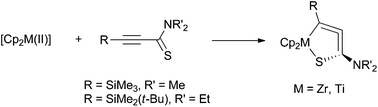
Org. Chem. Front., 2015,2, 681-687
https://doi.org/10.1039/C5QO00072F
A mechanistic study on the SHi reaction at tin atoms in a radical cascade reaction
A kinetic study on the intramolecular direct radical substitution reaction on tin atoms was undertaken.
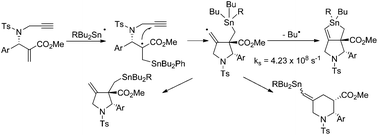
Org. Chem. Front., 2015,2, 713-720
https://doi.org/10.1039/C5QO00063G
1,3-Dipolar cycloaddition reactions of phthalic anhydrides with an azomethine ylide
A series of phthalic anhydrides underwent a 1,3-dipolar cycloaddition reaction with N-benzylazomethine ylide to produce unstable spiro(isobenzofuran-1,5′-oxazolidin)-3-ones, which underwent a subsequent reductive ring-opening reaction to afford 1(3H)-isobenzofuranones.
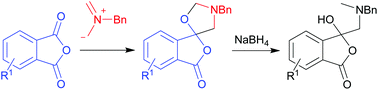
Org. Chem. Front., 2015,2, 705-712
https://doi.org/10.1039/C5QO00062A
Atroposelective synthesis of axially chiral P,S-ligands based on arynes
An unprecedented atropo-diastereoselective aryl–aryl coupling via arynes allows for the modular synthesis of enantiopure P,S-ligands.
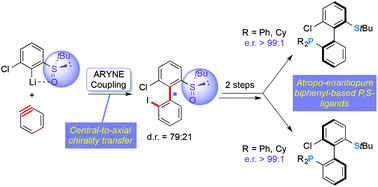
Org. Chem. Front., 2015,2, 634-644
https://doi.org/10.1039/C5QO00067J
An ortho-directed C–H borylation/Suzuki coupling sequence in the formation of biphenylbenzylic amines
ortho-C–H borylation of benzylic amines has been used in conjunction with a Suzuki–Miyaura coupling reaction to access biphenylbenzylic amines in good yields and requiring a single purification step.
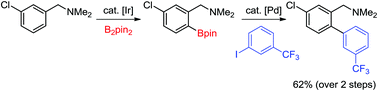
Org. Chem. Front., 2015,2, 661-664
https://doi.org/10.1039/C4QO00348A
Copper-catalysed cross-couplings of arylboronate esters with aryl and heteroaryl iodides and bromides
Cu-catalysed cross-coupling for mono- and di-arylations of aryl and heteroaryl iodides and bromides is achieved with arylboronate esters.
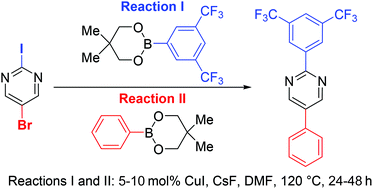
Org. Chem. Front., 2015,2, 649-653
https://doi.org/10.1039/C4QO00331D
Aerobic oxidation of cyclohexanones to phenols and aryl ethers over supported Pd catalysts
ZrO2 supported palladium catalysts promoted the aerobic oxidation of cyclohexanones to give phenols and aryl ethers.
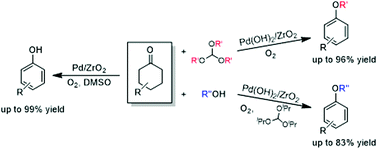
Org. Chem. Front., 2015,2, 654-660
https://doi.org/10.1039/C4QO00354C
Formal base-free homolytic aromatic substitutions via photoredox catalysis
We developed a simple and convenient method to assemble biaryls exploiting a photoredox catalyst and visible light.

Org. Chem. Front., 2015,2, 464-469
https://doi.org/10.1039/C5QO00031A
A one-pot dilithiation–lithium–zinc exchange–Negishi coupling approach to 2,6-di(hetero)aryl substituted dithienothiazines – a novel class of electronically fine-tunable redox systems
2,6-Di(hetero)aryl substituted dithienothiazines with fine-tunable electronic properties are efficiently accessible by lithiation–lithium–zinc exchange–Negishi cross-coupling in a one-pot fashion.
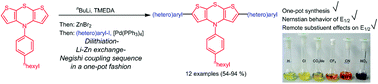
Org. Chem. Front., 2015,2, 481-491
https://doi.org/10.1039/C5QO00046G
Mechanistic studies and optimisation of a Pd-catalysed direct arylation reaction using phosphine-free systems
The mechanistic study of the initial step in direct arylation provides valuable insight for optimising reaction conditions.
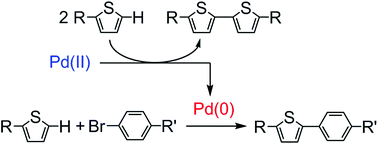
Org. Chem. Front., 2015,2, 520-525
https://doi.org/10.1039/C4QO00351A
An alkyne hydrosilylation–Hiyama coupling approach to highly functionalised 1,3-dienes
A high yielding and completely stereoselective hydrosilylation–Hiyama protocol has been established for the synthesis of highly functionalised E,E-dienes.
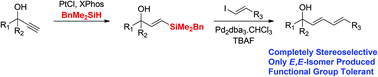
Org. Chem. Front., 2015,2, 510-514
https://doi.org/10.1039/C5QO00002E
Regio- and stereoselective synthesis of α-hydroxy-β-azido tetrazoles
Unreported α-hydroxy-β-azido tetrazoles are prepared in a single step and mild conditions from readily available epoxy nitriles.
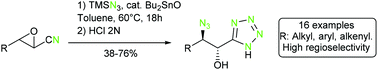
Org. Chem. Front., 2015,2, 492-496
https://doi.org/10.1039/C4QO00345D
A divergent synthesis of 3,10-dialkylpicenes
A series of 3,10-dialkylated picenes has been synthesized through sequential Ni-catalyzed alkynylation of a C–O bond, alkylation, and hydrogenation.
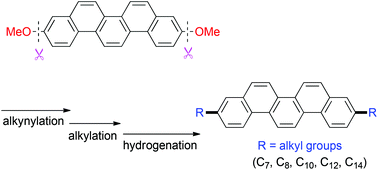
Org. Chem. Front., 2015,2, 536-541
https://doi.org/10.1039/C5QO00039D
New building blocks for iminosugars: a concise synthesis of polyhydroxylated N-alkoxypiperidines through an intramolecular azepine ring contraction
Polyhydroxylated piperidines are a functionally rich class of biologically active molecules that have broad therapeutic potential.
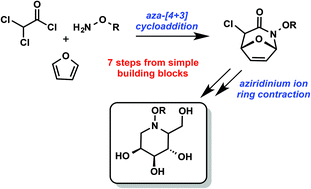
Org. Chem. Front., 2015,2, 497-501
https://doi.org/10.1039/C4QO00330F
Synthesis of bisarylethyne–peptide conjugates
Convenient preparation of bisarylethyne–peptide conjugates, and a mild procedure for the H- or D-reduction of the bisarylethyne triple bond.
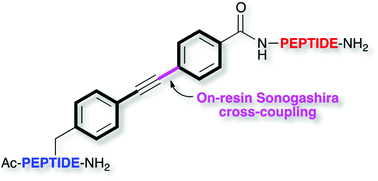
Org. Chem. Front., 2015,2, 531-535
https://doi.org/10.1039/C4QO00357H
An iodine-promoted Meyer–Schuster rearrangement for the synthesis of α-iodo unsaturated ketones
A facile and efficient iodine-promoted Meyer–Schuster rearrangement of propargyl alcohols for the synthesis of α-iodo-α,β-unsaturated ketones is presented.
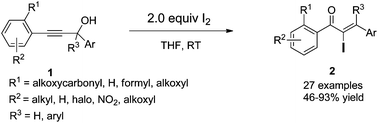
Org. Chem. Front., 2015,2, 506-509
https://doi.org/10.1039/C5QO00048C
A highly stereo-controlled protocol to prepare pipecolic acids based on Heck and cyclohydrocarbonylation reactions
A consecutive series of metal-catalyzed reactions for the preparation of enantiomerically pure piperidine derivatives.
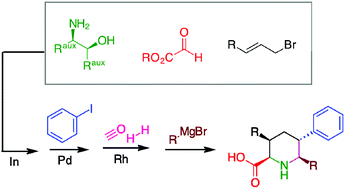
Org. Chem. Front., 2015,2, 526-530
https://doi.org/10.1039/C5QO00025D
Metal catalyzed cross-coupling of aryl and benzyl methyl sulfides: nickel catalyzed Caryl–Csp3 and Csp3–Csp3 bond formations
The nickel catalyzed functionalization of CAr–SMe and Csp3–SMe bonds by direct exchange of the sulfur atom with an activated sp3-carbon has been developed. The protocol allows the conversion of aryl and benzyl methyl sulfides to trimethylsilylated products in good yields.

Org. Chem. Front., 2015,2, 350-353
https://doi.org/10.1039/C5QO00001G
Synthetic strategies toward the decalin motif of maklamicin and related spirotetronates
Controlling the selectivity of an intramolecular Diels–Alder cycloaddition (IMDA) allows efficient synthetic access to the decalin motif of spirotetronates.
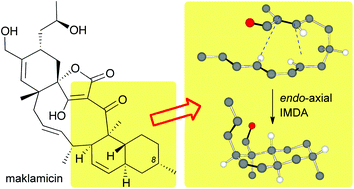
Org. Chem. Front., 2015,2, 388-393
https://doi.org/10.1039/C4QO00332B
Pd(II)-catalyzed asymmetric addition of arylboronic acids to cyclic N-sulfonyl ketimine esters and a DFT study of its mechanism
A highly efficient palladium-catalyzed asymmetric arylation of cyclic ketimine esters is developed, which provides the desired product in up to 99% yield with up to 99% ee. The mechanism of enantioselection is studied using DFT calculation.
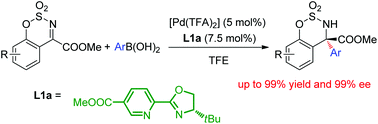
Org. Chem. Front., 2015,2, 398-402
https://doi.org/10.1039/C4QO00347K
Cleavage of the C–C triple bond of ketoalkynes: synthesis of 4(3H)-quinazolinones
A novel strategy to 4(3H)-quinazolinones from ketoalkynes and o-aminobenzamides through C–C triple bond fragmentation and two C–N bond formations under external oxidant and metal free conditions.
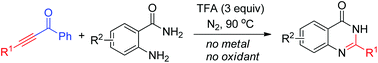
Org. Chem. Front., 2015,2, 366-368
https://doi.org/10.1039/C4QO00260A
Asymmetric synthesis of (αS)-polyfluoroalkylated N-Boc-prolinols by the diethyl zinc-induced asymmetric Meerwein–Ponndorf–Verley reduction of perfluoroalkyl N-Boc-pyrrolidyl ketones
Asymmetric synthesis of (αS)-polyfluoroalkylated prolinols by the asymmetric Meerwein–Ponndorf–Verley-type reduction of perfluoroalkyl N-Boc-pyrrolidyl ketones with Et2Zn has been developed.
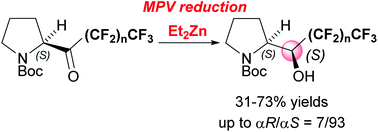
Org. Chem. Front., 2015,2, 369-371
https://doi.org/10.1039/C5QO00008D
Cationic iridium-catalyzed C–H alkylation of 2-substituted pyridine N-oxides with acrylates
The reaction of 2-arylmethyl-, 2-aryl-, and 2-alkyl substituted pyridine N-oxides with acrylates proceeded in the presence of a cationic Ir-rac-BINAP catalyst under heating conditions.
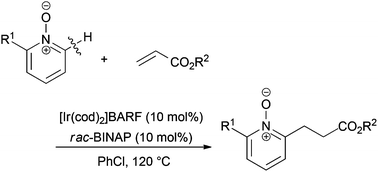
Org. Chem. Front., 2015,2, 383-387
https://doi.org/10.1039/C4QO00355A
Synthesis of (−)-mesembrine using the quaternary carbon-constructing allylic substitution
The allylic substitution for construction of quaternary carbons is used for synthesis of (−)-mesembrine.
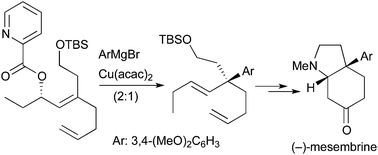
Org. Chem. Front., 2015,2, 328-335
https://doi.org/10.1039/C4QO00353E
Phase-transfer-catalyzed asymmetric desymmetrizations of cyclopentanones
Highly enantioselective phase-transfer-catalyzed epoxide-opening reaction and isomerization of cyclopentanones were achieved.
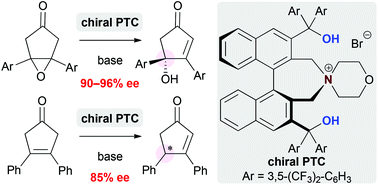
Org. Chem. Front., 2015,2, 336-339
https://doi.org/10.1039/C4QO00339J
Selective monoalkylation of amines with light electrophiles using a flow microreactor system
The challenging direct monoalkylation of primary and secondary amines with small triflates (C1 to C3) was accomplished in a flow microreactor by virtue of efficient mixing and short reaction times.
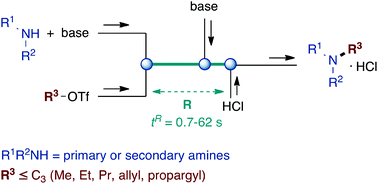
Org. Chem. Front., 2015,2, 324-327
https://doi.org/10.1039/C4QO00342J
CO-enabled rhenium hydride catalyst for directed C(sp2)–H bond alkylation with olefins
We report the first example of CO-enabled rhenium hydride complex-catalyzed inter- and intra-molecular hydroarylation of activated alkenes, with a broad reaction scope.
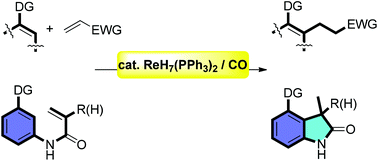
Org. Chem. Front., 2015,2, 378-382
https://doi.org/10.1039/C4QO00329B
Remarkable electron-withdrawing effect of the Ph2P(O)-ethynyl group: Ph2P(O)-ethynyl-substituted aryl halides and copper acetylides for tailor-made Sonogashira couplings
Ph2P(O)-ethynylphenyl bromide showed higher reactivity in Sonogashira couplings than phenylethynylphenyl bromide because of the electron-withdrawing nature of the Ph2P(O) group.
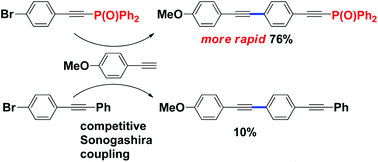
Org. Chem. Front., 2015,2, 248-252
https://doi.org/10.1039/C4QO00325J
4-Alkenyl-5H-1,2,3-oxathiazole 2,2-dioxides in catalytic and enantioselective [4 + 2] cycloaddition through iminium activation. Straightforward access to the trans-decaline framework and to densely functionalized cyclohexanes
4-Alkenyl-5H-1,2,3-oxathiazole 2,2-dioxides undergo Michael/Michael cascade reaction with enals through iminium/enamine activation.
![Graphical abstract: 4-Alkenyl-5H-1,2,3-oxathiazole 2,2-dioxides in catalytic and enantioselective [4 + 2] cycloaddition through iminium activation. Straightforward access to the trans-decaline framework and to densely functionalized cyclohexanes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4QO00316K&imageInfo.ImageIdentifier.Year=2015)
Org. Chem. Front., 2015,2, 206-210
https://doi.org/10.1039/C4QO00316K
Deuterative cyclization of sulfanyl 1,6-diynes: complete and monodeuteration of functional groups on heterocycles
Regioselective H/D exchange reaction of functional groups on heterocycles proceeded via a transition metal-free reductive cyclization of sulfanyl 1,6-diynes using sodium borodeuteride/ethanol-D1.
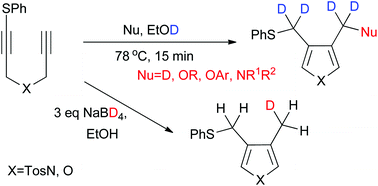
Org. Chem. Front., 2015,2, 201-205
https://doi.org/10.1039/C4QO00333K
Thieno[2,3,a]carbazole donor-based organic dyes for high efficiency dye-sensitized solar cells
A new class of D–π-A organic dyes based on thieno[2,3,a]carbazole as an electron donor showed high efficiencies for dye-sensitized solar cells.
![Graphical abstract: Thieno[2,3,a]carbazole donor-based organic dyes for high efficiency dye-sensitized solar cells](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4QO00285G&imageInfo.ImageIdentifier.Year=2015)
Org. Chem. Front., 2015,2, 253-258
https://doi.org/10.1039/C4QO00285G
N-heterocyclic carbene-based ruthenium-catalyzed direct amidation of aldehydes with amines
Ru-catalyzed dehydrogenative amide synthesis from aldehydes and amines was achieved, based on the idea of using a hemiaminal intermediate to generate the active Ru-hydride species.

Org. Chem. Front., 2015,2, 241-247
https://doi.org/10.1039/C4QO00319E
Co-catalysis between DABCO and a Brønsted acid in the catalytic [4 + 2] annulation of isatin with but-3-yn-2-one and mechanistic investigations
Catalytic amounts of the base catalyst DABCO in cooperation with a proton source afford the [4 + 2] cycloadducts of isatins with but-3-yn-2-one in moderate to good yields. Moreover, related plausible mechanisms have been proposed in detail based on control and deuterium labeling experiments.
![Graphical abstract: Co-catalysis between DABCO and a Brønsted acid in the catalytic [4 + 2] annulation of isatin with but-3-yn-2-one and mechanistic investigations](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4QO00323C&imageInfo.ImageIdentifier.Year=2015)
Org. Chem. Front., 2015,2, 211-215
https://doi.org/10.1039/C4QO00323C
Synthesis and characterization of N-2-aryl-1,2,3-triazole based iridium complexes as photocatalysts with tunable photoredox potential
N-2-Aryl chelated 1,2,3-triazole-Ir(III) complexes with various substituents were prepared for the first time.
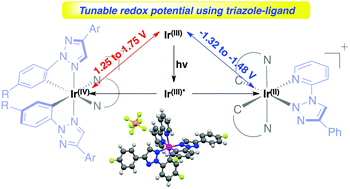
Org. Chem. Front., 2015,2, 141-144
https://doi.org/10.1039/C4QO00281D
Binary reducing agents containing dichloroindium hydride for the selective, partial, or tandem reductions of bifunctional compounds consisting of halo-nitriles, halo-esters and halo-carboxylic acids
Selective, partial, or tandem reductions of bifunctional organic halides is reported.
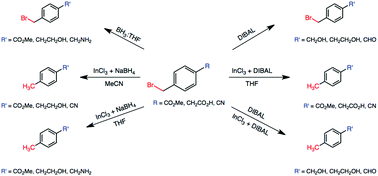
Org. Chem. Front., 2015,2, 133-140
https://doi.org/10.1039/C4QO00308J
Cationic Pd(II)-catalyzed cyclization of N-tosyl-aniline tethered alkynyl ketones initiated by hydropalladation of alkynes: a facile way to 1,2-dihydro or 1,2,3,4-tetrahydroquinoline derivatives
A cationic Pd(II)-catalyzed intramolecular alkyne-carbonyl reductive coupling reaction of N-tosyl-aniline tethered alkynyl ketones under transfer hydrogenation conditions to give hydroquinolines is developed.
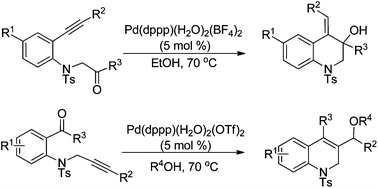
Org. Chem. Front., 2015,2, 145-149
https://doi.org/10.1039/C4QO00286E
A chiral bicyclo[2.2.2]octa-2,5-diene ligand substituted with the ferrocenyl group and its use for rhodium-catalyzed asymmetric 1,4-addition reactions
New chiral diene ligand, Fc,Ph-bod, showed higher enantioselectivity than Ph-bod in the rhodium-catalyzed asymmetric 1,4-addition of aryl- and alkenylboronic acids.
![Graphical abstract: A chiral bicyclo[2.2.2]octa-2,5-diene ligand substituted with the ferrocenyl group and its use for rhodium-catalyzed asymmetric 1,4-addition reactions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4QO00292J&imageInfo.ImageIdentifier.Year=2015)
Org. Chem. Front., 2015,2, 127-132
https://doi.org/10.1039/C4QO00292J
Copper(II)-catalyzed methoxylation of unactivated (hetero)aryl C–H bonds using a removable bidentate auxiliary
A copper-catalyzed methoxylation of unactivated (hetero)aryl C–H bonds has been developed.

Org. Chem. Front., 2015,2, 119-123
https://doi.org/10.1039/C4QO00276H
Chiroptical sensing of oligonucleotides with a cyclic octapyrrole
A cyclic octapyrrole shows a strong chiroptical response upon complexation with an oligonucleotide that has high thymine content.
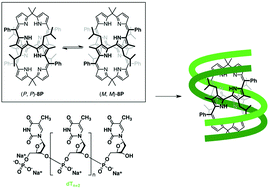
Org. Chem. Front., 2015,2, 29-33
https://doi.org/10.1039/C4QO00268G
Polycyclic imidazo[1,2-a]pyridine analogs – synthesis via oxidative intramolecular C–H amination and optical properties
Oxidative C–H amination has proved to be an efficient strategy to construct pyrido[2′,1′:2,3]imidazo[4,5-b]indoles and their π-expanded analogs.
![Graphical abstract: Polycyclic imidazo[1,2-a]pyridine analogs – synthesis via oxidative intramolecular C–H amination and optical properties](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4QO00248B&imageInfo.ImageIdentifier.Year=2015)
Org. Chem. Front., 2015,2, 21-28
https://doi.org/10.1039/C4QO00248B
Efficient access to 1H-indazoles via copper-catalyzed cross-coupling/cyclization of 2-bromoaryl oxime acetates and amines
A novel synthesis of 1H-indazoles via copper-catalyzed cross-coupling/cyclization of 2-bromoaryl oxime acetates and amines is reported.
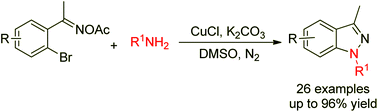
Org. Chem. Front., 2014,1, 1295-1298
https://doi.org/10.1039/C4QO00244J
Brønsted acid-catalyzed synthesis of carbazoles from 2-substituted indoles
A simple and efficient approach for the synthesis of disubstituted carbazoles has been developed from o-haloanilines and terminal alkynes using a two-step strategy, namely, Sonogashira coupling and intramolecular cyclization.
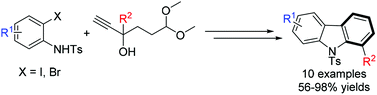
Org. Chem. Front., 2014,1, 1197-1200
https://doi.org/10.1039/C4QO00242C
Catalytic dehydrogenative aromatization: an alternative route to functionalized arenes
Catalytic dehydrogenative aromatization has emerged as an efficient and environmentally friendly way to access functionalized arenes in recent years.
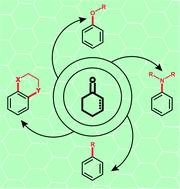
Org. Chem. Front., 2015,2, 279-287
https://doi.org/10.1039/C4QO00358F
About this collection
Professor Ei-ichi Negishi, the Noble laureate in 2010, will celebrate his 80th birthday in 2015. Organic Chemistry Frontiers is delighted to present an online themed collection dedicated to this special occasion. Guest Editors of this themed collection are Bruce H. Lipshutz (UC Santa Barbara) and
Tamotsu Takahashi (Hokkaido University).