A highly stereo-controlled protocol to prepare pipecolic acids based on Heck and cyclohydrocarbonylation reactions†‡
Abstract
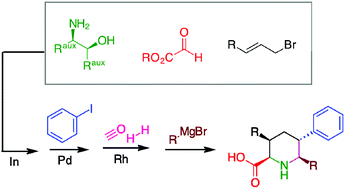
A novel synthetic protocol based on an indium-mediated glyoxylate allylation, Heck coupling and Rh-catalysed cyclohydrocarbonylation (CHC) was established to access enantiomerically pure polysubstituted pipecolic acids. The key steps are the Heck reaction, which is performed exclusively using a phenone-oxime derived palladacycle and the domino hydroformylation-cyclisation of a styryl derivative obtained from the Heck coupling. The reaction scheme, proceeding with good stereoselectivity, is also suitable for the preparation of substituted piperidine derivatives.
- This article is part of the themed collection: Celebrating the 80th Birthday of Professor Ei-ichi Negishi

 Please wait while we load your content...
Please wait while we load your content...