Silylative coupling of olefins with vinylsilanes in the synthesis of functionalized alkenes†
Abstract
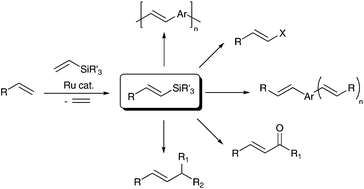
The development of highly selective methods for the synthesis of functionalized olefins, based on sequential catalytic reactions of organometallic reagents, has been the subject of extensive study because of their versatile applications in organic synthesis and materials science. The silylative coupling of olefins with vinyl-substituted organosilicon compounds (discovered in our group) represents one of the most efficient and straightforward methods for the synthesis of stereodefined alkenylsilanes and bis(silyl)alkenes, which are particularly attractive scaffolds for further transformations including palladium-catalyzed cross-coupling with organic halides (Hiyama coupling) or electrophile-induced desilylation. The article highlights the recent developments and covers the literature mainly from the last decade in the sequential (also one-pot) synthetic strategies including ruthenium-catalyzed silylative coupling followed by desilylative cross-coupling, acylation and halogenation, leading to stereodefined organic derivatives such as (E)-alkenyl halides, (E)-α,β-unsaturated ketones or arylene-(E)-vinylene derivatives which are widely applied as fine chemicals, functional materials or building blocks in organic synthesis.
- This article is part of the themed collections: 2015 Organic Chemistry Frontiers Review-type Articles and Celebrating the 80th Birthday of Professor Ei-ichi Negishi

 Please wait while we load your content...
Please wait while we load your content...