Regio- and stereoselective synthesis of α-hydroxy-β-azido tetrazoles†
Abstract
Unreported α-hydroxy-β-azido tetrazoles were prepared in one step from readily available α,β-epoxy nitriles. This reaction involves a dibutyltin oxide-catalyzed cycloaddition of the nitrile reacting with TMSN3 leading to the tetrazole moiety, and opening of the epoxide by the azide anion. High levels of regio- and stereoselectivity are obtained in this reaction and are discussed, also by means of quantum mechanical DFT calculations. The azido group in these compounds could be uneventfully reduced to the corresponding amine thus leading to an α-hydroxy-β-amino tetrazole, surrogate of the corresponding carboxylic acid, while reaction with triphenylphosphine led to propargylic amines.
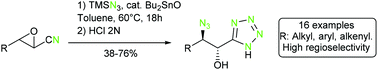
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers in 2015 and Celebrating the 80th Birthday of Professor Ei-ichi Negishi

 Please wait while we load your content...
Please wait while we load your content...